CV benefit of PCSK9 inhibitor is consistent regardless of inflammation level
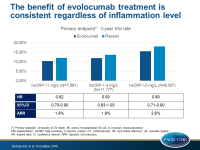
ACC 2018 A prespecified FOURIER-analysis showed that evolocumab decreases CV events across hsCRP strata, with greater absolute risk reductions in patients with higher baseline hsCRP.
Inflammatory and Cholesterol Risk in the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Patients With Elevated Risk)Literature - Bohula EA, Giugliano RP, Leiter LA, et al. - Circulation 2018;137, and simultaneously presented at ACC.18 in Orlando, FL, USA
Introduction and methods
The prognostic value of LDL-c and high-sensitivity C-reactive protein (hsCRP) has been established, but not in the setting of extremely low LDL-c [1-3]. In this analysis of the FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Patients With Elevated Risk) trial, the consistency of benefit of the PCSK9 inhibitor evolocumab for the prevention of cardiovascular (CV) events by baseline hsCRP was assessed. In addition, the importance of inflammatory and residual cholesterol risk as defined by LDL-c and hsCRP levels was evaluated.
FOURIER was a randomized, double-blind placebo-controlled trial that enrolled 27,564 patients aged 40 to 85 years with clinically evident, stable atherosclerotic CV disease and additional risk factors [4,5]. Eligible patients had LDL-c ≥70 mg/dL or non–HDL-c ≥100 mg/dL while taking an optimized regimen of lipid-lowering therapy (at least atorvastatin 20 mg daily or its equivalent, with or without ezetimibe).
Patients were randomly assigned to subcutaneous evolocumab (either 140 mg Q2W or 420 mg Q4W) or matching placebo injections, and were followed for a median of 2.2 years (IQ: 1.8–2.5 years). The primary efficacy endpoint was a composite of CV death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization. The key secondary endpoint was a composite of CV death, MI, or stroke.
For this analysis, endpoints were compared across strata of baseline hsCRP (<1, 1–3, and >3 mg/dL), and assessed across values for baseline hsCRP and LDL-c achieved at 1-month in the entire trial population.
Main results
- Out of 27,495 patients, 29% had a low baseline hsCRP level (<1 mg/L), 41% had an intermediate hsCRP (1–3 mg/L), and 30% had a high hsCRP (>3 mg/L).
- The median baseline hsCRP level was 1.7 mg/L (IQR: 0.9–3.6) in the evolocumab arm and 1.8 mg/L (IQR: 0.9–3.6) in the placebo arm.
- The change in hsCRP at 48 weeks was –0.2 mg/L (IQR: –1.0 to 0.4) in both treatment arms with no significant difference in achieved hsCRP levels between evolocumab and placebo (median 48-week hsCRP level: 1.4 mg/L; IQR: 0.7–3.1 for both treatments).
- The relative risk reduction with evolocumab versus placebo for the primary endpoint was consistent across baseline hsCRP strata (HR for hsCRP <1 mg/L: 0.82; 95%CI: 0.70–0.95; HR for hsCRP 1–3 mg/L: 0.93; 95%CI: 0.83–1.05; HR for hsCRP >3 mg/L: 0.80; 95%CI: 0.71–0.90; P-interaction=0.17).
- The ARRs for the primary endpoint with evolocumab were 1.6% (95%CI: –0.5 to 3.7), 1.8% (95%CI: 0.0 - 3.5) and 2.6% (95%CI: 0.4 - 4.9), respectively, in those with baseline hsCRP levels of <1, 1 to 3, and >3 mg/L.
- After adjustment for confounders and hsCRP levels, a higher LDL-c concentration achieved at 1-month was independently associated with higher rates of the primary and key secondary endpoints, with a 9% (HR: 1.09; 95%CI: 1.05–1.14; P<0.0001) and 12% (HR: 1.12; 95%CI: 1.06–1.18; P <0.0001) increased relative risk, respectively, for every doubling in LDL-c.
- After adjustment for confounders and achieved LDL-c, a higher hsCRP level was independently associated with higher rates of the primary and key secondary end points, with a 9% (HR: 1.09; 95%CI: 1.07–1.12; P<0.0001) and 13% (HR: 1.13; 95%CI: 1.09–1.17; P<0.0001) increased relative risk, respectively, for every doubling in hsCRP.
Conclusion
LDL-c reduction with evolocumab decreases CV events across hsCRP strata in FOURIER with greater absolute risk reductions in patients with higher baseline hsCRP. Adverse CV event rates were independently associated with both LDL-c and hsCRP, which was a risk predictor even in patients with very low levels of LDL-c. These results support the importance of inflammatory and residual cholesterol risk.
References
1. Bohula EA, Giugliano RP, Cannon CP, et al. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation. 2015;132:1224–1233.
2. Ridker PM, Cannon CP, Morrow D, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28.
3. Morrow DA, de Lemos JA, Sabatine MS, et al. Clinical relevance of C-reactive protein during follow-up of patients with acute coronary syndromes in the Aggrastat-to-Zocor Trial. Circulation. 2006;114:281–288.
4. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722.
5. Sabatine MS, Giugliano RP, Keech A, et al. Rationale and design of the Further cardiovascular Outcomes Research with PCSK9 Inhibition in subjects with Elevated Risk trial. Am Heart J. 2016;173:94–101