Different statins may have different renal effects in diabetic patients with proteinuria
03/03/2015
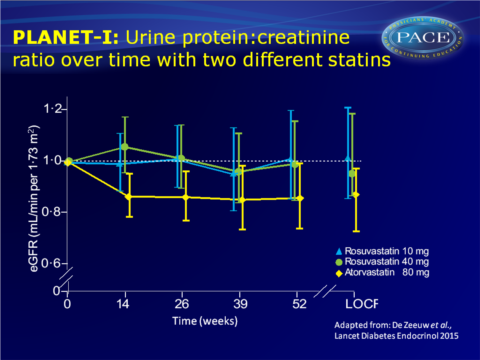
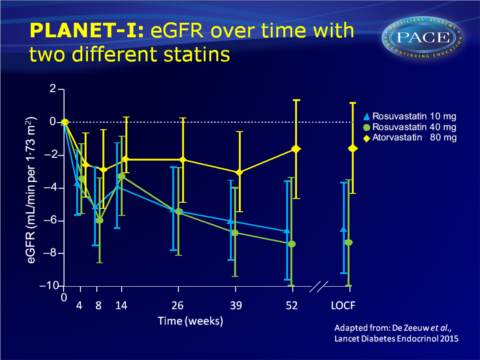
PLANET I study showed lower protein urinary secretion but unaffected eGFR after 1 year atorvastatin 80 mg, while protein excretion was unchanged but eGFR reduced with rosuvastatin 40 mg.
Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): a randomised clinical trial.Literature - de Zeeuw D et al., Lancet Diabetes Endocrinol. 2015
de Zeeuw D, Anzalone DA, Cain VA et al.,
Lancet Diabetes Endocrinol. 2015 Mar;3(3):181-90. doi: 10.1016/S2213-8587(14)70246-3.
Background
Declining renal function progressing to end-stage renal disease is increasingly seen worldwide, often as a consequence of atherosclerosis and type 2 diabetes (T2DM) [1]. Lifestyle changes, glucose-lowering and blood pressure-lowering drugs can diminish the risk of adverse renal outcomes, and also cholesterol is thought to be a risk factor for renal failure.Although experimental studies suggest that cholesterol contributes to renal disease progression, and that lowering cholesterol concentrations with statins was renoprotective [2], the effect of statins on renal function is less clear in clinical studies. A combination of simvastatin and ezetimibe recently failed to show a renoprotective effect in a hard renal outcome study [3,4].
The Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients with Progressive Renal Disease (PLANET I) trial was set up to assess the renal effects of 52 weeks of treatment with atorvastatin 80 mg (n=111), rosuvastatin 10 mg (n=118) or rosuvastatin 40 mg (n=124) in 353 patients with diabetes (type 1 [n=47] or 2 [n=278] in the intention-to-treat population, n=325) and proteinuria (urine protein:urine creatinine [U-PCR] 500–5000 mg/g). PLANET II was a similar trial in patients with proteinuria but without diabetes.
Main results
- Only in the atorvastatin 80 mg group, U-PCR was significantly lower at 52 weeks, than at baseline (change in U-PCR: 0.87, 95%CI: 0.77-0.99, P=0.033). U-PCR was also significantly lower at week 14, 26 and 39 with atorvastatin 80 mg.
- Urine albumin:urine creatinine ratio (UACR) was significantly lower at week 52 (change: 0.82, 95%CI: 0.71-0.95, P=0.011) and all other time points with atorvastatin 80 mg, and at week 52 with rosuvastatin 40 mg (change: 0.84, 95%CI: 0.70-0.99, P=0.041).
- eGFR was significantly lower in the rosuvastatin 10 mg (-3.70, 95%CI: -6.49 to -0.91, P=0.0098) and 40 mg group (-7.29, 95%CI: -11.0 to -3.59, P=0.0002), while it did not change in the atorvastatin 80 mg group.
- Since data of PLANET II were similar to PLANET I, the data were combined. This showed a significant difference in change in U-PCR between the atorvastatin 80 mg and the rosuvastatin 10 mg and 40 mg groups. eGFR was significantly greater with rosuvastatin than in the atorvastatin 80 mg group.
Conclusion
The PLANET I study showed reduced urinary protein excretion, but unaffected eGFR in patients with diabetes and proteinuria who were treated during 1 year with atorvastatin 80 mg. In patients randomised to rosuvastatin 40 mg, urinary protein excretion was not changed, but eGFR decreased as compared to baseline.Both PLANET I and II suggest, although they were not powered to directly compare the two statins, that atorvastatin and rosuvastatin have different renal profiles in patients with proteinuria. Based on the current study, it cannot definitely be concluded whether atorvastatin is more renoprotective or neutral, and rosuvastatin neutral or harmful in this high-risk population.
Editorial comment [5]
“Recent findings have raised concerns about potential renal adverse effects of some statins, particularly when given in high doses.” (…) In the randomised PLANET I study, “Acute kidney injury and doubling of serum creatinine concentration were more common among patients taking rosuvastatin 40 mg than among those taking rosuvastatin 10 mg or atorvastatin 80 mg. The type of renal injury induced by rosuvastatin was not established. However, some case reports indicate that rosuvastatin can induce interstitial nephritis.The PLANET studies have several limitations. Neither PLANET I nor PLANET II was powered to assess differences between treatment groups for any outcome. Indeed, in PLANET I, although the primaryoutcome fell significantly in the atorvastatin group but not in the two rosuvastatin groups, post-hocbetween-group comparisons were not statistically significant. Thus, further research is needed to establish whether atorvastatin provides any benefit relative to rosuvastatin.”
Find this article at The Lancet
References
1 Gansevoort RT, van der Heij B, Stegeman CA, et al. Trends in the incidence of treated end-stage renal failure in The Netherlands: hope for the future? Kidney Int Suppl 2004; 92: S7–S10.2 Campese VM, Park J. HMG-CoA reductase inhibitors and the kidney. Kidney Int 2007; 71: 1215–22.
3 Baigent C, Landray MJ, Reith C, et al. The eff ects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377: 2181–92.
4 Haynes R, Lewis D, Emberson J, et al; SHARP Collaborative Group. Effects of lowering LDL cholesterol on progression of kidney disease. J Am Soc Nephrol 2014; 25: 1825–33.
5 Camplese VM. Statins and the kidney: friend or foe? Lancet Diabetes Endocrinol. February 4, 2015 http://dx.doi.org/10.1016/S2213-8587(14)70260-8
