DPP-4 inhibition may reduce carotid atherosclerosis in T2DM patients in primary prevention
DPP-4 inhibition with sitagliptin for 24 months decreased the intima-media thickness of the internal carotid artery in type 2 diabetic patients without established CVD.
Sitagliptin on carotid intima-media thickness in type 2 diabetes patients receiving primary or secondary prevention of cardiovascular disease: A subgroup analysis of the PROLOGUE studyLiterature - Tanaka A, Yoshida H, Nanasato M, et al. - Int J Cardiol 2018; published online ahead of print
Introduction and methods
Studies evaluating the ability of dipeptidyl peptidase-4 (DPP-4) inhibitors to decrease carotid intima-media thickness (IMT) have come to conflicting results [1-3]. A reasonable explanation for this might be that relevant studies enrolled different types of type 2 diabetes mellitus (T2DM) patients; the PROLOGUE study [1] enrolled patients with and without established CVD, whereas others included only those without CV events.
In this post hoc analysis of the PROLOGUE study [1], it was evaluated whether the effect of sitaglitin on carotid atherosclerosis differed between primary and secondary prevention groups.
PROLOGUE was a 2-year, prospective, randomized, open-label study, comparing the effect of sitagliptin with standard of care on carotid IMT progression in 442 T2DM patients. In this subanalysis of the study, the effect on sitagliptin and conventional therapy on changes in carotid -IMT measurements at 24 months were compared, in groups stratified according to presence or absence of previous CV events.
Main results
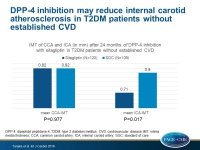
- Baseline-adjusted mean common carotid artery (CCA)-IMT at 24 months was 0.82 mm in both groups (mean difference: 0.000 mm; 95%CI: −0.022 to 0.022; P = 0.977) in the primary prevention subgroup, and 0.84 vs. 0.85 mm (mean difference: − 0.010 mm; 95%CI: −0.035 to 0.014; P=0.392) in the secondary prevention subgroup.
- Changes in mean and max internal carotid artery (ICA)-IMT at 24 months in the primary prevention subgroup were significantly lower in the sitagliptin group compared with the conventional therapy group (0.71 vs. 0.80; mean difference: −0.096 mm; 95%CI: −0.175 to −0.018; P=0.017; max difference: − 0.162 mm; 95%CI: −0.272 to −0.052; P=0.004).
Conclusion
DPP-4 inhibition with sitagliptin for 24 months decreased ICA-IMT in T2DM patients in primary prevention, although it did not decrease CCA-IMT neither in primary, nor in secondary prevention.
References
1. Oyama J, Murohara T, Kitakaze M, et al, PROLOGUE study investigators. The effect of sitagliptin on carotid artery atherosclerosis in type 2 diabetes: The PROLOGUE randomized controlled trial, PLoS Med 2016 ;13:e1002051.
2. Mita T, Katakami N, Yoshii H, et al. Collaborators on the Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A) Trial, Alogliptin, a dipeptidyl peptidase 4 inhibitor, prevents the progression of carotid atherosclerosis in patients with type 2 diabetes: the study of preventive effects of alogliptin on diabetic atherosclerosis (SPEAD-A). Diabetes Care 2016; 39:139–148.
3. Mita T, Katakami N, Shiraiwa T, et al, Collaborators on the Sitagliptin Preventive Study of Intima-Media Thickness Evaluation (SPIKE) Trial, Sitagliptin attenuates the progression of carotid intima-media thickening in insulin-treated patients with type 2 diabetes: the sitagliptin preventive study of intima-media thickness evaluation (SPIKE): a randomized controlled trial. Diabetes Care 2016;39:455–464.