Dual GLP-1 and glucagon receptor agonist improves glycemic control and reduces body weight
In a phase 2a study, MEDI0382 improved glycemic control and reduced body weight in patients with stable type 2 diabetes who were overweight or obese.
MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a studyLiterature - Ambery P, Parker VE, Stumvoll M, et al. - Lancet 2018; 391: 2607–18
Introduction and methods
Weight loss improves glycemic control and reduces cardiovascular risk in overweight and obese patients with type 2 diabetes (T2DM) [1]. Existing glucose-lowering medications have a neutral effect on weight, or lead to a modest degree of weight loss, whereas bariatric surgery leads to effective glycemic control, but is associated with adverse effects and high costs [2,3]. MEDI0382 is a dual GLP-1 and glucagon receptor agonist, which is developed to improve glycemic control and to lead to clinically meaningful weight loss through insulin-tropic effects on appetite and energy expenditure. Although glucagon mobilizes hepatic glucose, another peptide with GLP-1 and glucagon receptor dual agonist activity was thought to promote normoglycemia through the equipoise of the two receptor activities. MEDI0382 is thought to partially mimic the hormonal environment after bariatric surgery, and in preclinical studies, it promoted more weight loss than GLP-1 receptor agonism alone [4].
This randomized, placebo-controlled, double-blind study, consisting of a multiple-ascending dose (MAD) phase, and a phase 2a, evaluated the optimal dosing regimen, efficacy, tolerability, and safety of MEDI0382 in overweight or obese T2DM patients. For this purpose, stable T2DM patients aged 18-65 years, with HbA1c of 6.5–8.5% at screening and a BMI of 27-40 kg/m² were recruited. Patients were randomly assigned to receive placebo or MEDI0382 once-daily subcutaneous before breakfast or after 8 hours of fasting. In the MAD phase of the study (N=61), 5 different study drug regimens were tested, ranging from 100 μg for 7 days to 300 μg for 22 days.
In the phase 2a part of the study (N=51), individuals received study drug at a dose of up to 200 μg for a maximum of 41 days or placebo in a 1:1 fashion, followed by a safety follow-up of 28 days. The endpoints were the percentage change in the glucose area under the curve at 0–4 h (AUC0–4 h) after a mixed-meal tolerance test (MMTT), and the change in body weight from baseline to the end of treatment. Moreover, the changes in fasting plasma glucose concentrations and HbA1c levels were assessed, as well as changes in liver fat, subcutaneous adipose tissue, and visceral adipose tissue. Adverse events were self-reported.
Main results
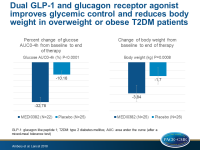
- MEDI0382 showed a significant decrease in percentage glucose AUC0–4 h post MMTT compared with placebo (meanLS: –32.78%; 90%CI: –36.98 to –28.57 vs –10.16%; 90%CI: –14.10 to –6.21; P<0.0001). The mean difference between groups was –22·62% (90%CI: –28.40 to –16.85).
- There was a significant decrease in body weight with MEDI0382 compared with placebo (meanLS: –3.84 kg; 90%CI: –4·55 to –3·12 vs –1.70 kg; 90%CI: –2·40 to –1·01; P=0.0008). The mean difference between groups was 2.14 kg (90%CI: –3.13 to –1.31).
- Fasting plasma glucose concentrations were significantly reduced in the MEDI0382 group compared with the placebo group (meanLS: –2.8 mmol/L; 90%CI: –3.2 to –2.4 vs –1.1 mmol/L; 90%CI: –1·4 to –0·7; P<0.0001).
- HbA1c levels were significantly lowered in the MEDI0382 group compared with the placebo group (meanLS: –0.9%; 90%CI: –1.0 to –0.8 vs –0·6%; 90%CI: –0.7 to –0.5; P=0.0004).
- Participants treated with MEDI0382 had a significant reduction in liver fat compared with placebo (RR: –39.12% vs –19.51%). Subcutaneous and visceral adipose tissue were also reduced in patients on MEDI0382.
- Gastrointestinal disorders including nausea, vomiting, and decreased appetite, as well as headache, occurred more frequently with MEDI0382 compared with placebo. In the phase 2a part of the study, adverse events leading to study discontinuation were observed in 3 patients on MEDI0382 (12%) and 1 patient on placebo (4%).
- Cmax, AUC0-inf, AUC0-tau and CL/F values were highest in patients after treatment with at least 200 µg of MEDI0382 for 17 days or more (MAD and phase 2a).
Conclusion
In stable overweight or obese T2DM patients, MEDI0382 led to clinically meaningful reductions in blood glucose and body weight compared with placebo.
Discussion
MEDI0382 contains a long-acting GLP-1 receptor agonist, which is thought to be more effective in reducing fasting glucose concentration via glucose-dependent insulinotropic and glucagonostatic effects than short-acting GLP-1 receptor agonists that predominantly lower postprandial glucose concentration through delayed gastric emptying.
Editorial comment
In their editorial article, Rayner and Horowitz [5] discuss the limitations of the Ambery et al study, which include that there was no active comparator as is common in early-phase trials, due to which it is unknown how much was gained by glucagon receptor agonism over stimulation of the GLP-1 receptors alone. The improvements seen in glucose levels and body weight were in the same range as those achieved by GLP-1 receptor agonists. Moreover, the main adverse effects were self-reported, and energy balance as well as liver inflammation and fibrosis were not assessed. The authors conclude: ‘The outcomes of the study by Ambery and colleagues support the ongoing development of MEDI0382, but a comprehensive understanding of mechanisms will be pivotal to successful therapeutic developments based on the gut–pancreas hormone axis. In this area, whether two (or more) will prove to be better than one remains to be determined’.
References
1. Franz MJ, Boucher JL, Rutten-Ramos S, et al. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet 2015; 115: 1447–63.
2. Piya MK, Tahrani AA, Barnett AH. Emerging treatment options for type 2 diabetes. Br J Clin Pharmacol 2010; 70: 631–44.
3. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292: 1724–37.
4. Henderson SJ, Konkar A, Hornigold DC, et al. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes Metab 2016; 18: 1176–90.
5. Rayner CK and Horowitz M. Agonism of receptors in the gut–pancreas axis in type 2 diabetes: are two better than one? Lancet 2018; 391: 2577-8.