Dual RAAS-blockade therapy worsens renal disease in diabetics
VA NEPHRON-D: RAAS-blockade combination therapy tips the risk/benefit-balance towards higher risk in diabetic nephropathy, without a benefit for the primary efficacy outcome.
Combined Angiotensin Inhibition for the Treatment of Diabetic NephropathyLiterature - Fried et al., NEJM 2013 - N Engl J Med November 9, 2013
Fried LF, Emanuele N, Zhang JH et al.; for the VA NEPHRON-D Investigators
N Engl J Med November 9, 2013DOI: 10.1056/NEJMoa1303154
Background
Patients with diabetes and proteinuria are at high risk of developing end-stage renal disease (ESRD) [1]. Progression of proteinuric kidney disease may be inhibited by blockade of the renin-angiotensin system (RAAS)s [2-4]. The degree of reduction in proteinuria correlates with the slope of the decrease in the glomerular filtration rate (GFR) [1,5]. Therefore, it has been hypothesised that further lowering of proteinuria may reduce the risk of progression [5]. A larger decrease in proteinuria can be achieved with combination therapy with an angiotensin-converting-enzyme (ACE) inhibitor and a angiotensin II-receptor blocker (ARB), than with either monotherapy [6].The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) found no cardiovascular (CV) or renal benefits in patients at high CV risk , when comparing combination therapy with monotherapy. An increased risk of hyperkalemia and acute kidney injury requiring dialysis was seen [7,8].
The Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) study was a double-blind randomised controlled study that compared the safety and efficacy of combination therapy with an ACE inhibitor and an ARB, and ARB monotherapy in slowing the progression of proteinuric diabetic nephropathy.
-- The trial was prematurely stopped (after a median follow-up of 2.2 years) as recommended by the data and safety monitoring committee, as a result of safety concerns due to increased rates of serious adverse events such as hyperkalemia and acute kidney injury, in the combination therapy group, in comparison to the monotherapy group, and little evidence for an effect on the primary endpoint.--
Main results
- 152 primary endpoint events (first occurrence of a decline in the estimated GFR, ESRD, or death) were seen in the monotherapy group (21.0%, event rate 10.8 events/100 person years (py)), and 132 events in the combination therapy group (18.2%, 9.5 events/100 py). There was no significantly different event risk between the two treatment groups.
- The monotherapy group showed 101 secondary endpoint events (decrease in estimated GFR or ESRD)(14.0%) and 77 in the combination therapy group (10.6%), yielding overall event rates of respectively 7.2 per 100 py and 5.5 per 100 py.
- No statistically significant differences in the rate of CV events were seen between the two treatments.
- No significant difference was seen in the decline of estimate GFR between the groups( −2.7 ml per minute per 1.73 m2 per year in the combination therapy group and −2.9 ml per minute per 1.73 m2 per year in the monotherapy group, P=0.17).
- More patients on combination therapy suffered from serious adverse events than patients on monotherapy (98 events per 100 py vs. 82 per 100 py), and the proportion of serious adverse events attributable to study medication was higher in the combination group.
Acute kidney injury was the main driving factor of the higher rate of serious adverse events in the combination therapy group (12.2 vs. 6.7 events per 100 py, HR: 1.7, 95%CI: 1.3-2.2, P<0.001).
The risk of hyperkalaemia was also higher in the combination group: HR: 2.8 (95%CI: 1.8-4.3, P<0.001, 6.3 vs. 2.6 events per 100 py).
Download pace-NEJM Fried 2013.pptx
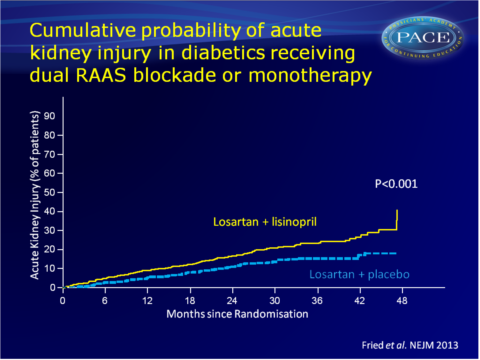
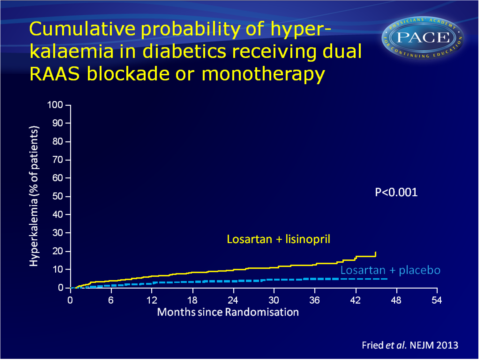
Conclusion
Combination therapy with an ACE inhibitor and an ARB, as compared with therapy with an ARB alone, was associated with a higher risk of serious adverse events, mostly acute kidney injury and hyperkalaemia. Combination therapy did not give a significant benefit with respect to progression of renal disease or death. Conditional power calculations suggested that no statistically significant benefit would have been achieved if the study were completed as planned.Editorial comment [9]
It has been hypothesised that blocking more than one step in the RAAS would better yield better organ protection, since monotherapy gives incompletely RAAS blockade. Indeed, further lowering of blood pressure and albuminuria have been observed with different dual RAAS blockade strategies and clinicians have started using dual RAAS blockade. However, hard-outcome trials should guide decisions to use dual RAAS blockade. In addition to ONTARGET and ALTITUDE, VA-NEPHRON-D now demonstrated that dual therapy does not decrease CV and renal morbidity, but also that it is associated with increased risk.The conclusion of these trials goes beyond the decision not to use dual RAAS blockade in diabetic patients. The results also demonstrate that an improvement of surrogate markers like blood pressure and albuminuria does not translate into risk reduction, because in contrast to the monotherapy studies, the protective effects do no longer outweigh the extra risk.
Find this article online
References
1. de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004;65:2309-20.
2. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861-9.
3. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting– enzyme inhibition on diabetic nephropathy. N Engl J Med 1993;329:1456- 62. [Erratum, N Engl J Med 1993;330:152.]
4. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin- receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851-60.
5. de Zeeuw D. Albuminuria, not only a cardiovascular/renal risk marker, but also a target for treatment? Kidney Int Suppl 2004;92:S2-S6.
6. Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med 2008;148:30-48.
7. Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008;372:547-53.
8. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547-59.
9. De Zeeuw, D. The End of Dual Therapy with Renin–Angiotensin–Aldosterone System Blockade? N Engl J Med Nov 9 2013
