Dual RAS blockade is not recommended
03/02/2013
Because of a comparable mortality rate and an increased risk of adverse events, the use of dual RAS blockade instead of monotherapy is not recommended.
AbstractLiterature - Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. - BMJ. 2013 Jan 28;346:f360. doi: 10.1136/bmj.f360.
Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials.
Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH.
BMJ. 2013 Jan 28;346:f360. doi: 10.1136/bmj.f360.
Background
An experimental model showed a “synergistic” effect between angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). This observation formed the basis of the concept of dual blockade of the renin-angiotensin system (RAS). Although solid evidence on the safety and efficacy of dual RAS blockade was lacking, this type of therapy has been mentioned in several guidelines [1-3]. Dual therapy was commonly used in patients with hypertension and/or with diabetes or proteinuria and – to a lesser extent – in those with refractory heart failure. In the US, more than 200,000 patients are currently treated with dual RAS blockade, mostly by the combination of an ARB and ACE inhibitor (70%) [4,5] However, the long term efficacy and safety of dual blockade is not well defined.A systematic review and meta-analysis of total 33 RCTs (n = 68,405; mean age 61 years, 71% men+ mean duration of 52 weeks) compared the long term efficacy and adverse events of dual RAS blockade (any two of ACE inhibitors, ARBs, or aliskiren) with monotherapy.
Main results
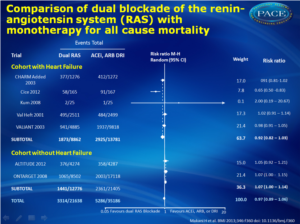
Compared with monotherapy, dual RAS blockade was:- not associated with any significant benefit for all cause mortality (relative risk 0.97, 95% CI 0.89-1.06) and cardiovascular mortality (0.96, 0.88-1.05).
- associated with an 18% reduction in admissions to hospital for heart failure (0.82, 0.74-0.92).
- associated with a 55% increase in the risk of hyperkalaemia (P<0.001), a 66% increase in the risk of hypotension (P<0.001), a 41% increase in the risk of renal failure (P=0.01), and a 27% increase in the risk of withdrawal owing to adverse events (P<0.001).
Conclusion
The risk-to-benefit ratio argues against the use of dual blockade of the renin-angiotensin system instead of monotherapy. Although dual therapy may have seemingly beneficial effects on certain surrogate endpoints, it failed to reduce mortality and was associated with an increased risk of adverse events, such as hyperkalemia, hypotension, and renal failure compared with monotherapy.
References
1. Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009;119:1977-2016.2. McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;14:803-69.
3. Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl 1):S11-63.
4. Banerjee S, Kaye S. The role of targeted therapy in ovarian cancer. Eur J Cancer 2011;47(Suppl 3):S116-30.
5. Messerli FH, Staessen JA, Zannad F. Of fads, fashion, surrogate endpoints and dual RAS blockade. Eur Heart J 2010;31:2205-8.Objectives:
To compare the long term efficacy and adverse events of dual blockade of the renin-angiotensin system with monotherapy.
Methods:
Systematic review and meta-analysis.PubMed, Embase, and the Cochrane central register of controlled trials, January 1990 to August 2012.Randomised controlled trials comparing dual blockers of the renin-angiotensin system with monotherapy, reporting data on either long term efficacy (≥1 year) or safety events (≥4 weeks), and with a sample size of at least 50. Analysis was stratified by trials with patients with heart failure versus patients without heart failure.
Results:
33 randomised controlled trials with 68 405 patients (mean age 61 years, 71% men) and mean duration of 52 weeks were included. Dual blockade of the renin-angiotensin system was not associated with any significant benefit for all-cause mortality (relative risk 0.97, 95% confidence interval 0.89 to 1.06) and cardiovascular mortality (0.96, 0.88 to 1.05) compared with monotherapy. Compared with monotherapy, dual therapy was associated with an 18% reduction in admissions to hospital for heart failure (0.82, 0.74 to 0.92). However, compared with monotherapy, dual therapy was associated with a 55% increase in the risk of hyperkalaemia (P<0.001), a 66% increase in the risk of hypotension (P<0.001), a 41% increase in the risk of renal failure (P=0.01), and a 27% increase in the risk of withdrawal owing to adverse events (P<0.001). Efficacy and safety results were consistent in cohorts with and without heart failure when dual therapy was compared with monotherapy except for all-cause mortality, which was higher in the cohort without heart failure (P=0.04 v P=0.15), and renal failure was significantly higher in the cohort with heart failure (P<0.001 v P=0.79).
Conclusions:
Although dual blockade of the renin-angiotensin system may have seemingly beneficial effects on certain surrogate endpoints, it failed to reduce mortality and was associated with an excessive risk of adverse events such as hyperkalaemia, hypotension, and renal failure compared with monotherapy. The risk to benefit ratio argues against the use of dual therapy.