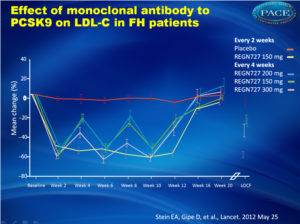
Effect of monoclonal antibody to PCSK9 on LDL-C in FH patients
This phase 2 study assessed the LDL-C efficacy and safety of REGN727 in various doses and dosing intervals in patients with hypercholesterolaemia treated with statin with or without ezetimibe.
Effect of a monoclonal antibody to PCSK9, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial.Literature - Stein EA et al, Lancet. 2012 May 25
Stein EA, Gipe D, et al.
Lancet. 2012 May 25.
Background
Aggressive LDL-C reduction in familial hypercholesterolaemia (FH) is associated with improved rates of cardiovascular disease [1-3]. For patients with heterozygous FH unable to achieve LDL-C goals on optimal medical treatment, there is an unmet medical need for additional, effective LDL-C lowering therapies.A potential approach is to inhibit PCSK9, to increase residual LDL receptor activity. Serum PCSK9 has an active role in controlling the expression of LDL receptors by reducing their recycling by binding to the receptor along with LDL and targeting it for lysosomal destruction [4].
REGN727/SAR236553 (REGN727) is a full human monoclonal antibody targeted to PCSK9, inhibiting binding to the LDL receptor [5]. This phase 2 study assessed the LDL-C efficacy and safety of REGN727 in various doses and dosing intervals in patients with hypercholesterolaemia treated with statin with or without ezetimibe. Patients were randomly assigned to one of four active dose regimens of REGN727 or placebo.
Main results
After 12 weeks, LDL-C levels in patients on REGN727 were reduced by between 28.9 and 67.9%, depending on dosing regimen, compared with 10.7% among those given placebo. The reductions in LDL-C were rapid, reaching a maximum at 2 weeks (fig. 1) for every dose but the 150 mg every 2 weeks, which showed a continued decrease with subsequent dosing, stabilizing after the third dose. Dosing every 4 weeks achieved similar reductions to the 150 mg every 2 weeks dose at 2 weeks, but the LDL-C reduction was not fully maintained over the full 4-week period.Numbers of patients achieving the prespecified goals of < 2.6 mmol/L and < 1.8 mmol/L were highest in the group of 150 mg every 2 weeks (94% and 81%, respectively), compared to placebo (13% and 0%, respectively). The most common adverse event was injection-site reaction.
Conclusion
Subcutaneous administration of REGN727 may provide a new option, on top of existing therapies, to lower LDL-C and reach LDL-C goals in patients with severe heterozygous FH.
References
1. Scientific Steering Committee on behalf of the Simon Broome Register Group. Mortality in treated heterozygous familial hypercholesterolemia: implications for clinical management. Atherosclerosis 1999; 142: 105–12.
2. Ellis A, Zhou R, Stein EA. Effect of lipid-lowering treatment on natural history of heterozygous familial hypercholesterolemia in past three decades. Am J Cardiol 2011; 108: 223–26.
3. Raal FJ, Santos RD, Blom DJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind,placebo-controlled trial. Lancet 2010; 375: 998–1006.
4. Qian YW, Schmidt RJ, Zhang Y, et al. Secreted PCSK9 downregulates low density lipoprotein receptor through receptor-mediated endocytosis. J Lipid Res 2007; 48: 1488–98.
5. Chan JC, Piper DE, Cao Q, et al. A proprotein convertase subtilisin/ kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc Natl Acad Sci USA 2009; 106: 9820–25.
Abstract
Background:Inhibition of proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) resulted in large reductions of low-density lipoprotein cholesterol (LDL-C) in phase 1 trials. We assessed the efficacy and safety of various doses and dosing intervals of REGN727, a monoclonal antibody to PCSK9, added to statins, to further lower LDL-C in patients with heterozygous familial hypercholesterolaemia.
Methods:
This multicentre, randomised, placebo-controlled phase 2 trial was done at 16 lipid clinics in the USA and Canada. Between Jan 18, 2011, and Nov 7, 2011, we enrolled adults with heterozygous familial hypercholesterolaemia and LDL-C concentrations of 2•6 mmol/L or higher on stable diet and statin dose, with or without ezetimibe. Patients were randomly assigned to receive REGN727 150 mg, 200 mg, or 300 mg every 4 weeks, or 150 mg every 2 weeks, or placebo every 2 weeks (ratio 1:1:1:1:1). Randomisation was stratified by concomitant use of ezetimibe at baseline. Investigators, study staff, and patients were masked to treatment group. Blinding was maintained by administration of placebo alternating with REGN727 for the groups of 4 week dosing. The primary endpoint was mean percent reduction in LDL-C from baseline at week 12 and was analysed in the modified intention-to-treat population with an analysis of covariance (ANCOVA) model with treatment group. This trial is registered in clinicaltrials.gov, number NCT 01266876.
Findings:
77 patients were randomly assigned to study groups (15-16 patients per group) and all were analysed. Least-squares (LS) mean LDL-C reduction from baseline to week 12 was 28•9% (SE 5•08) for 150 mg every 4 weeks (p=0•0113), 31•54% (4•91) for 200 mg every 4 weeks (p=0•0035), 42•53% (5•09) for 300 mg every 4 weeks (p<0•0001), and 67•90% (4•85) for 150 mg every 2 weeks (p<0•0001), compared with 10•65% (5•04) with placebo. One serious adverse event was reported with placebo and none with REGN727. No increases of more than three times the upper limit of normal were reported for hepatic transaminases or creatinine kinase. The most common adverse event was injection-site reaction with one patient in the group of 300 mg REGN727 terminating treatment.
Interpretation:
REGN727 was well tolerated and achieved substantial further LDL-C reduction in patients with heterozygous familial hypercholesterolaemia and elevated LDL-C treated with high-dose statins, with or without ezetimibe. REGN727 has the potential to provide optimum control of LDL-C in patients with this disorder.