Empagliflozin safely improves glycaemic control, with lower insulin need
Lower HbA1c levels and weight loss were achieved with addition of empagliflozin, despite needing lower insulin doses, in difficult-to-treat obese, T2DM patients.
Improved Glucose Control With Weight Loss, Lower Insulin Doses, and No Increased Hypoglycemia With Empagliflozin Added to Titrated Multiple Daily Injections of Insulin in Obese Inadequately Controlled Type 2 DiabetesLiterature - Rosenstock J et al., Diabetes Care. 2014 - Diabetes Care. 2014 Jun 14
Rosenstock J, Jelaska A, Frappin G, et al.; on behalf of the EMPA-REG MDI Trial Investigators
Diabetes Care. 2014 Jun 14. pii: DC_133055. [Epub ahead of print]
Background
Almost half of the patients with type 2 diabetes (T2DM) do not achieve glycaemic control with basal insulin plus oral antidiabetes agents after 24 weeks of treatment [1]. Intensification of the insulin regimen can be achieved with progressive additions of prandial insulin up to multiple daily injections of insulin (MDI insulin). Some patients still do not achieve glycaemic control because they are insulin resistant, or other comorbidities such as obesity may further complicate obtaining glycaemic control with high insulin doses[2].Empagliflozin is a potent and selective sodium glucose cotransporter 2 (SGLT2) inhibitor [3], that reduces renal glucose reabsorption, which leads to urinary glucose excretion [4], thereby reducing hyperglycaemia. Phase III studies have shown that empagliflozin improved glycaemic control, reduced body weight and blood pressure, both as monotherapy and as add-on therapy to different glucose-lowering strategies [5-8]. Since empagliflozin functions independently of beta-cell function [9], it induces weight loss and does not confer a high risk of hypoglycaemie [5-8], it may be an attractive option to use in combination with insulin.
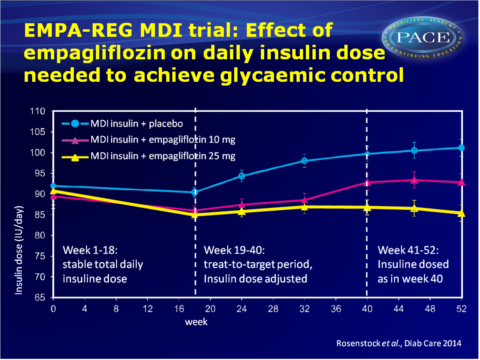
The EMPA-REG MDI trial evaluated the efficacy and safety of 52 weeks of empagliflozin 10 mg and 25 mg vs. placebo as add-on therapy to MDI insulin with or without metformin in obese patients with T2DM and insufficient glycaemic control. During the first 18 weeks, the total daily dose of insulin had to remain within 10% of the prescribed dose at randomisation. Week 19-40 served as treat-to-target period, in which insulin dose was adjusted to achieve a preprandial glucose target of <5.5 mmol/L and a postprandial glucose target of <7.8 mmol/L. For weeks 41-52, the total daily dose of insulin had to remain within 10% of the dose prescribed at week 40 (adjustments for safety reasons were allowed).
Main results
- At week 18, adjusted mean (+SE) change from baseline in HbA1c was -0.50 + 0.05% with placebo as compared with -0.94 + 0.05% with empagliflozin 10 mg and -1.02 + 0.05% with 25 mg (P<0.001 for both compared with placebo).
- At week 18, adjusted mean (+SE) change from baseline in fasting plasma glucose (FPG) was 0.19 +0.16 mmol/L with placebo, and 0.98 + 0.16 mmol/L with empagliflozin 10 mg and -1.36 + 0.16 mmol/L with empagliflozin 25 mg (P<0.001 vs. placebo for both).
- While body weight increased with placebo (0.34+ 0.18 kg), it decreased with empagliflozin 10 mg (-0.97 +0.18 kg) and 25 mg (-1.54 + 0.18 kg)(P<0.001 vs placebo for both).
- Adjusted mean +SE change from baseline in HbA1c was -0.81 +0.08% in the placebo group, as compared to the empagliflozin groups (10 mg: -1.18 +0.08%, 25 mg: -1.27+0.08%), which used much lower titrations of insulin.
- More than 30% of patients on empagliflozin 10 mg and over 40% of patients on 25 mg who had HbA1c>7% at the start of the study, reached HbA1c < 7%, as compared to 21% of patients in the placebo group.
- Mean +SE changes from baseline in FPG at week 52 were -0.63 + 0.19 mmol/L with placebo, as compared with -0.63 + 0.19 mmol/L with empagliflozin 10 mg (P=0.012) and -1.43 + 0.19 mmol/L with empagliflozin 25 mg (P=0.004).
- Body weight was significantly reduced with empagliflozin 10 mg (-1.95 +0.36 kg) and 25 mg (-2.04+0.36 kg), as compared with an increase of 0.44 +0.36 kg with placebo.
- During the 52 weeks of treatment, the proportion of people experiencing adverse events (AE), serious AE or AE leading to discontinuation were similar across treatment groups. In the first 18 weeks, the proportion of patients with hypoglycaemic AEs was somewhat higher in empagliflozin-treated patients (39.8% (10 mg) and (25 mg) 41.3% vs. 37.2% in placebo), but over the full 52 week period including the treat-to-target period, no difference was seen between treatment groups in proportion of patients with hypoglycaemic AEs (51.1% (10 mg) and 57.7% (25 mg) vs. 58.0% (placebo)).
Download Rosenstock Diab Care 2014 PACE.pptx
Conclusion
This study shows that, compared with insulin titrations alone, addition of empagliflozin 10 mg or 25 mg once daily for 52 weeks improved glycaemic control in difficult-to-treat obese, insulin-resistant patients. Lower HbA1c levels were achieved with addition of empagliflozin, despite needing lower insulin doses, and with weight loss and no increase in the risk of hypoglycaemia. Empagliflozin in addition to MDI insulin was well tolerated, thus may provide a new treatment option in this difficult to treat T2DM population.Find this article on Pubmed
References
1. RiddleMC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–3086
2. Pickup JC, Renard E. Long-acting insulin analogs versus insulin pump therapy for the treatment of type 1 and type 2 diabetes. Diabetes Care 2008;31(Suppl. 2):S140–S145
3. Grempler R, Thomas L, Eckhardt M, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab 2012;14:83–90
4. Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab 2013; 15:613–621
5. Roden M, Weng J, Eilbracht J, et al.; EMPAREG MONO Trial Investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2013; 1:208–219
6. Häring H-U, Merker L, Seewaldt-Becker E, et al.; EMPA-REG MET Trial Investigators. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, doubleblind, placebo-controlled trial. Diabetes Care 2014;37:1650–1659
7. Häring H-U, Merker L, Seewaldt-Becker E, et al.; EMPA-REG METSU Trial Investigators. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2013;36:3396–3404
8. Kovacs CS, Seshiah V, Swallow R, et al.; EMPA-REG PIO Trial Investigators. Empagliflozin improves glycaemic and weight control as addon therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab 2014;16:147–158
9. Rosenstock J, Jelaska A, Wang F, et al.; EMPA-REG BASAL Trial Investigators. Empagliflozin as add-on to basal insulin for 78 weeks improves glycemic control with weight loss in insulin-treated type 2 diabetes (T2DM) (Abstract). Diabetes 2013;62(Suppl. 1):A285
10. Kim Y, Babu AR. Clinical potential of sodiumglucose cotransporter 2 inhibitors in the management of type 2 diabetes. Diabetes Metab Syndr Obes 2012;5:313–327
