EXAMINE: CV outcomes with alogliptin in patients with diabetes and ACS
22/12/2011
EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome
EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE):News - Dec. 22, 2011
A cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome.
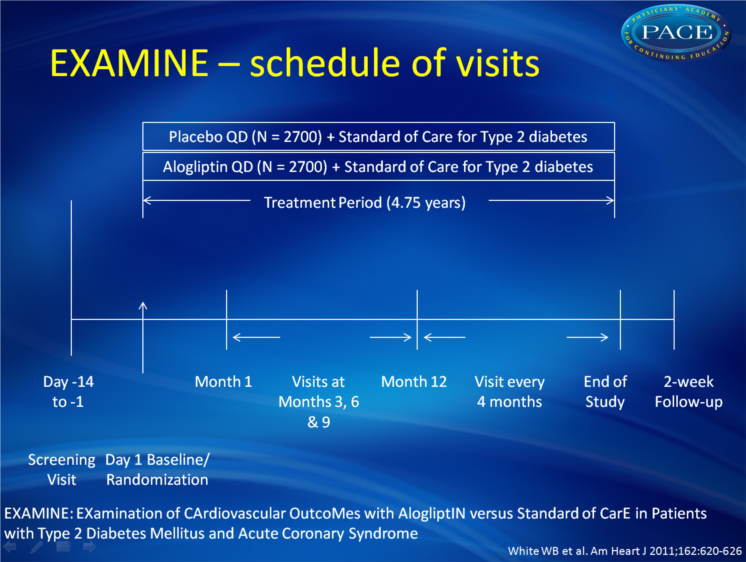
White WB, Bakris GL, Bergenstal RM, Cannon CP, Cushman WC, Fleck P, Heller S, Mehta C, Nissen SE, Perez A, Wilson C, Zannad F.
Am Heart J. 2011 Oct;162(4):620-626
Abstract
Comprehensive safety evaluation of new drugs for diabetes mellitus is needed in the area of cardiovascular (CV) outcomes, particularly in populations with high CV risk. Alogliptin, a dipeptidyl peptidase 4 inhibitor, is under development for the treatment of type 2 diabetes mellitus alone or in combination with other antidiabetic therapies. Long-term CV safety of alogliptin is being established in a randomized, placebo-controlled clinical study in patients with acute coronary syndrome (ACS) using an analytical approach that has both an interim and final assessment. The primary CV end point for this trial is a composite of CV death, nonfatal myocardial infarction, and nonfatal stroke. Approximately 5,400 men and women with type 2 diabetes and ACS (acute myocardial infarction or unstable angina) are being recruited and will be followed up for up to 4.5 years postrandomization. The statistical plan for the trial uses a design that evaluates the hazard ratio (HR) of alogliptin to placebo first based on the primary CV composite end point after accrual of 80 to 150 primary CV events and again when there are 550 to 650 primary CV events. In the first series of analyses, the upper bound of a group-sequential 1-sided repeated CI for the HR must be ≤1.8 for registration in the United States. At end of study, the upper bound of a subsequent group-sequential 1-sided repeated CI for the HR must be ≤1.3. For both group sequential analyses, the repeated CIs are calculated to insure simultaneous coverage probabilities of 97.5% for the true HR. Study progress: More than 2,000 ACS patients were randomized as of June 2011. EXAMINE will define the CV safety profile of this dipeptidyl peptidase 4 inhibitor in patients at high risk for CV events.Background
Type 2 diabetes mellitus is associated with microvascular and macrovascular complications [1]. In people with diabetes, the risk of cardiovascular disease is 2 to 4 times higher compared with individuals without diabetes [2]. It has been shown that improving glycemic control reduces microvascular risk, but a favorable impact on macrovascular events has not yet been determined.As antidiabetic agents might be associated with adverse CV outcomes, for new antidiabetic therapies a CV safety assessment has to be performed before and after approval.
A new antidiabetic treatment under development for type 2 diabetes is the dipeptidyl peptidase 4 (DPP-4) inhibitor alogliptin. By inhibiting DPP-4 and thereby preventing the rapid degradation of GLP-1, alogliptin stimulates control of elevated blood glucose by triggering pancreatic insulin secretion and suppression of pancreatic glucagon secretion [3]. Alogliptine, given either alone or in combination with other antidiabetic agents, lowers HbA1c levels by 0.6% to 1.0% in patients with type 2 diabetes mellitus, thereby rarely inducing hypoglycemia [3]. In a phase 3 study with alogliptin the CV event rate was too low to rule out a concern in patients with higher baseline risk [4].
The EXAMINE trial studied patients with type 2 diabetes with much higher CV risk in acute coronary syndrome (ACS) to establish the CV safety of alogliptin in this patient group.
Study design
EXAMINE is a phase 3, multicenter, prospective, double-blind randomized trial in which alogliptin isbeing compared with placebo on CV outcomes in approximately 5,400 patients with type 2 diabetes and a well-defined ACS event. The primary objective of EXAMINE is to demonstrate the noninferiority of major adverse cardiovascular events (MACE) on alogliptin versus placebo in the treatment of type 2 diabetes in a high-risk CV patient group.
The EXAMINE trial was initiated in 2009; at present more than 2.300 patients have been randomized into the trial and recruitment is still ongoing.
In summary, EXAMINE is an important study to establish the CV safety of alogliptin in patients with type 2 diabetes and ACS and will provide essential information on the safety of alogliptin in patients with type 2 diabetes who are at increased CV risk.
References
1. Meigs JB. Epidemiology of cardiovascular complications in type 2 diabetes mellitus. Acta Diabetol 2003;40(suppl 2):S358-61.2. Centers for Disease Control and Prevention (CDC). National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Atlanta, GA: US Dept of Health and Human Services, Centers for Disease Control and Prevention; 2008. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf.
3. Ghatak SB, Patel DS, Shanker N, et al. Alogliptin: a novel molecule for improving glycemic control in type II diabetes mellitus. Curr Diabetes Rev 2010;6:410-21.
4. White WB, Gorelick P, Fleck P, et al. Cardiovascular events in patients receiving alogliptin: findings from a pooled analysis of randomized clinical trials. (abstract)Meeting of the 70th Annual American Diabetes Association, Orlando, Florida, June 2; 2010.