Exenatide or glimepiride after metformin in type 2 diabetes
14/06/2012
Although metformin is first-line therapy for patients with type 2 diabetes, glycemic control fails with metformin alone in many patients. An open-label randomized controlled trial was conducted, which compared add-on exenatide with add-on glimepiride in patients who had failed to achieve glycemic control with metformin alone.
Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial.Literature - Gallwitz B, Guzman J, Dotta F, et al.; Lancet. 2012 Jun 8
Gallwitz B, Guzman J, Dotta F, et al.
Lancet. 2012 Jun 8. [Epub ahead of print]
Although metformin is first-line therapy for patients with type 2 diabetes [1,2], glycemic control fails with metformin alone in many patients. Glimepiride is a sulfonylurea that is commonly prescribed for such patients [1,3,4]. The risk of hypoglycaemia can restrict doses used in clinical practice [5]. Exenatide is a glucagon-like peptide-1 (GLP-1) agonist improving glycaemic control with glucose-dependent stimulation of insulin secretion without increased risk of hypoglycaemia. An open-label randomized controlled trial was conducted at 128 centers in 14 countries, which compared add-on exenatide with add-on glimepiride in patients who had failed to achieve glycemic control with metformin alone. A total of 515 patients were randomized to exenatide twice daily, 514 were put on glimepiride; the intent-to-treat analysis included 490 and 487 patients, respectively.
The primary outcome was the time to inadequate glycemic control and the need for alternative treatment, defined as an HbA1c of more than 9% after 3 months, or an HbA1c higher than 7% at two consecutive visits 3 months apart.
Strengths of the study included its long-term follow-up and its comparison between frequently used agents. Few studies have compared anti-diabetic drugs for the durability of their effectiveness on glycemic control, cost, quality of life, and their effects on late diabetic complications. GLP-1 receptor agonists have thus far displayed cardioprotective effects and reduced markers of inflammation, although regulators are watching closely for upcoming results on cardiovascular risks with these agents in the near future.
1. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32: 193–203
2. Holman RR, Paul SK, Bethel MA Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–89.
3. International Diabetes Federation. Global guideline for type 2 diabetes. 2005. http://www.idf.org/guidelines/type-2-diabetes (accessed Jan 19, 2012).
4. International Diabetes Federation. Treatment algorithm for people with type 2 diabetes. 2011. http://www.idf.org/treatment-algorithmpeople-type-2-diabetes (accessed Jan 19, 2012).
5. Kahn SE, Haff ner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427–43.
6. Drucker DJ, Sherman IS, Gorelick FS, et al. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefi ts. Diabetes Care 2010;33: 428–33.
7. Tahrani AA, Bailey CJ, Del Prato S, Barnett AH. Management of type 2 diabetes: new and future developments in treatment. Lancet 2011; 378: 182–97.
8. Madsbad S. Type 2 diabetes: which drug as add-on to metformin? Lancet. 2012 Jun 8. [Epub ahead of print]
BACKGROUND:
In people with type 2 diabetes, a dipeptidyl peptidase-4 (DPP-4) inhibitor is one choice as second-line treatment after metformin, with basal insulin recommended as an alternative. We aimed to compare the efficacy, tolerability, and safety of insulin glargine and sitagliptin, a DPP-4 inhibitor, in patients whose disease was uncontrolled with metformin.
METHODS:
In this comparative, parallel, randomised, open-label trial, metformin-treated people aged 35-70 years with glycated haemoglobin A(1c) (HbA(1c)) of 7-11%, diagnosis of type 2 diabetes for at least 6 months, and body-mass index of 25-45 kg/m(2) were recruited from 17 countries. Participants were randomly assigned (1:1) to 24-week treatment with insulin glargine (titrated from an initial subcutaneous dose of 0•2 units per kg bodyweight to attain fasting plasma glucose of 4•0-5•5 mmol/L) or sitagliptin (oral dose of 100 mg daily). Randomisation (via a central interactive voice response system) was by random sequence generation and was stratified by centre. Patients and investigators were not masked to treatment assignment. The primary outcome was change in HbA(1c) from baseline to study end. Efficacy analysis included all randomly assigned participants who had received at least one dose of study drug and had at least one on-treatment assessment of any primary or secondary efficacy variable. This trial is registered at ClinicalTrials.gov, NCT00751114.
FINDINGS:
732 people were screened and 515 were randomly assigned to insulin glargine (n=250) or sitagliptin (n=265). At study end, adjusted mean reduction in HbA(1c) was greater for patients on insulin glargine (n=227; -1•72%, SE 0•06) than for those on sitagliptin (n=253; -1•13%, SE 0•06) with a mean difference of -0•59% (95% CI -0•77 to -0•42, p<0•0001). The estimated rate of all symptomatic hypoglycaemic episodes was greater with insulin glargine than with sitagliptin (4•21 [SE 0•54] vs 0•50 [SE 0•09] events per patient-year; p<0•0001). Severe hypoglycaemia occurred in only three (1%) patients on insulin glargine and one (<1%) on sitagliptin. 15 (6%) of patients on insulin glargine versus eight (3%) on sitagliptin had at least one serious treatment-emergent adverse event.
INTERPRETATION:
Our results support the option of addition of basal insulin in patients with type 2 diabetes inadequately controlled by metformin. Long-term benefits might be expected from the achievement of optimum glycaemic control early in the course of the disease.
Lancet. 2012 Jun 8. [Epub ahead of print]
Background
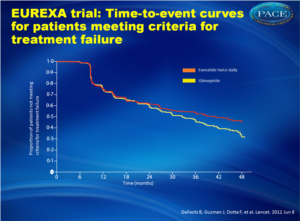
Although metformin is first-line therapy for patients with type 2 diabetes [1,2], glycemic control fails with metformin alone in many patients. Glimepiride is a sulfonylurea that is commonly prescribed for such patients [1,3,4]. The risk of hypoglycaemia can restrict doses used in clinical practice [5]. Exenatide is a glucagon-like peptide-1 (GLP-1) agonist improving glycaemic control with glucose-dependent stimulation of insulin secretion without increased risk of hypoglycaemia. An open-label randomized controlled trial was conducted at 128 centers in 14 countries, which compared add-on exenatide with add-on glimepiride in patients who had failed to achieve glycemic control with metformin alone. A total of 515 patients were randomized to exenatide twice daily, 514 were put on glimepiride; the intent-to-treat analysis included 490 and 487 patients, respectively.The primary outcome was the time to inadequate glycemic control and the need for alternative treatment, defined as an HbA1c of more than 9% after 3 months, or an HbA1c higher than 7% at two consecutive visits 3 months apart.
Main results
- In the exenatide group, 203 of 490 patients (41%) experienced treatment failure, compared with 262 patients of 487 (54%) in the glimepiride group (risk difference, 12.4; 95% confidence interval [CI], 6.2 to 18.6; hazard ratio, 0.748 [95% CI, 0.623 to 0.899]; P = .002).
- Significantly more patients on exenatide achieved an HbA1c lower than 7% (45% versus 31%, P<0.0001) and 6.5% or less (29% versus 18%, P=0.0001).
- Exenatide patients also had a significantly greater decrease in body weight - losing 3.32 kg compared with a gain of 1.15 kg for those on glimepiride (P<0.0001) - and significantly less hypoglycemia (P<0.0001).
- More patients on exenatide had adverse events, predominantly gastrointestinal effects such as nausea and diarrhea, and discontinued therapy. Most adverse events occurred within the first 6 months of treatment (P=0.0005).
Conclusion
Twice-daily exenatide appeared more beneficial than usual care with glimepiride in preventing the deterioration of glycemic control in patients with type 2 diabetes.
Editorial comment [8]
Strengths of the study included its long-term follow-up and its comparison between frequently used agents. Few studies have compared anti-diabetic drugs for the durability of their effectiveness on glycemic control, cost, quality of life, and their effects on late diabetic complications. GLP-1 receptor agonists have thus far displayed cardioprotective effects and reduced markers of inflammation, although regulators are watching closely for upcoming results on cardiovascular risks with these agents in the near future.
References
1. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32: 193–2032. Holman RR, Paul SK, Bethel MA Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–89.
3. International Diabetes Federation. Global guideline for type 2 diabetes. 2005. http://www.idf.org/guidelines/type-2-diabetes (accessed Jan 19, 2012).
4. International Diabetes Federation. Treatment algorithm for people with type 2 diabetes. 2011. http://www.idf.org/treatment-algorithmpeople-type-2-diabetes (accessed Jan 19, 2012).
5. Kahn SE, Haff ner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427–43.
6. Drucker DJ, Sherman IS, Gorelick FS, et al. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefi ts. Diabetes Care 2010;33: 428–33.
7. Tahrani AA, Bailey CJ, Del Prato S, Barnett AH. Management of type 2 diabetes: new and future developments in treatment. Lancet 2011; 378: 182–97.
8. Madsbad S. Type 2 diabetes: which drug as add-on to metformin? Lancet. 2012 Jun 8. [Epub ahead of print]
Abstract
BACKGROUND:In people with type 2 diabetes, a dipeptidyl peptidase-4 (DPP-4) inhibitor is one choice as second-line treatment after metformin, with basal insulin recommended as an alternative. We aimed to compare the efficacy, tolerability, and safety of insulin glargine and sitagliptin, a DPP-4 inhibitor, in patients whose disease was uncontrolled with metformin.
METHODS:
In this comparative, parallel, randomised, open-label trial, metformin-treated people aged 35-70 years with glycated haemoglobin A(1c) (HbA(1c)) of 7-11%, diagnosis of type 2 diabetes for at least 6 months, and body-mass index of 25-45 kg/m(2) were recruited from 17 countries. Participants were randomly assigned (1:1) to 24-week treatment with insulin glargine (titrated from an initial subcutaneous dose of 0•2 units per kg bodyweight to attain fasting plasma glucose of 4•0-5•5 mmol/L) or sitagliptin (oral dose of 100 mg daily). Randomisation (via a central interactive voice response system) was by random sequence generation and was stratified by centre. Patients and investigators were not masked to treatment assignment. The primary outcome was change in HbA(1c) from baseline to study end. Efficacy analysis included all randomly assigned participants who had received at least one dose of study drug and had at least one on-treatment assessment of any primary or secondary efficacy variable. This trial is registered at ClinicalTrials.gov, NCT00751114.
FINDINGS:
732 people were screened and 515 were randomly assigned to insulin glargine (n=250) or sitagliptin (n=265). At study end, adjusted mean reduction in HbA(1c) was greater for patients on insulin glargine (n=227; -1•72%, SE 0•06) than for those on sitagliptin (n=253; -1•13%, SE 0•06) with a mean difference of -0•59% (95% CI -0•77 to -0•42, p<0•0001). The estimated rate of all symptomatic hypoglycaemic episodes was greater with insulin glargine than with sitagliptin (4•21 [SE 0•54] vs 0•50 [SE 0•09] events per patient-year; p<0•0001). Severe hypoglycaemia occurred in only three (1%) patients on insulin glargine and one (<1%) on sitagliptin. 15 (6%) of patients on insulin glargine versus eight (3%) on sitagliptin had at least one serious treatment-emergent adverse event.
INTERPRETATION:
Our results support the option of addition of basal insulin in patients with type 2 diabetes inadequately controlled by metformin. Long-term benefits might be expected from the achievement of optimum glycaemic control early in the course of the disease.