Fewer CV events and mortality with GLP-1 analogue in patients with type 2 diabetes
ADA 2016 In T2DM patients at high CV risk, liraglutide on top of standard therapy was associated with lower rates of CV events and mortality, compared with placebo in the LEADER outcome trial.
Liraglutide and Cardiovascular Outcomes in Type 2 DiabetesLiterature - Marso SP et al., NEJM 2016
Marso SP, Daniels GH, Brown‑Frandsen K, et al.
N Engl J Med 2016;published online ahead of print
Background
Type 2 diabetes mellitus (T2DM) is characterised by hyperglycaemia and associated with a high risk of cardiovascular (CV), microvascular, and other complications. Treating hyperglycaemia leads to a decrease in the risk of microvascular complications, but there is uncertainty about the CV safety of anti-hyperglycaemic therapies, which has resulted to mandatory CV safety assessments of new T2DM therapies by regulatory authorities [1-3].Liraglutide is an analogue of human glucagonlike peptide 1 (GLP-1) that leads to decreases in glucose levels, body weight and blood pressure, as well as to increased pulse rate, which has been approved for the treatment of T2DM [4-6].
In this double-blind study (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results [LEADER] trial), the long-term efficacy and safety of liraglutide was evaluated in 9340 T2DM patients at high CV risk. Median time of exposure to liraglutide or placebo was 3.5 years, during a median follow-up time of 3.8 years in both groups.
Main results
- The composite primary outcome (first occurrence of death from CV causes, non-fatal myocardial infarction (MI), or non-fatal stroke) occurred in 608 of 4668 patients [13.0%] in the liraglutide group vs. 694 of 4672 [14.9%] in the placebo group (HR: 0.87; 95% CI: 0.78 - 0.97; P<0.001 for non-inferiority; P = 0.01 for superiority).
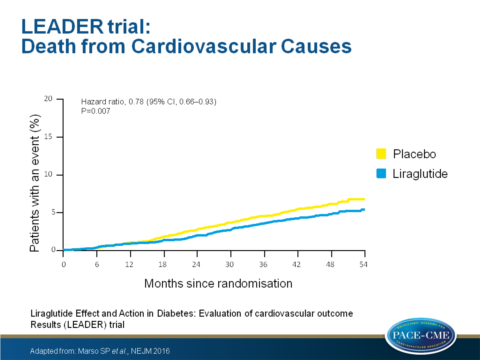
- Death from CV causes occurred in 219 patients [4.7%] in the liraglutide group vs. 278 [6.0%] in the placebo group (HR: 0.78; 95% CI: 0.66 - 0.93; P = 0.007).
- Death from any cause occurred in 381 patients [8.2%] in the liraglutide group vs. 447 [9.6%] in the placebo group (HR: 0.85; 95% CI: 0.74 - 0.97; P = 0.02).
- The frequencies of non-fatal MI and non-fatal stroke were lower in the liraglutide group than in the placebo group, although the differences were not significant.
- A significant interaction was observed with eGFR ≥ 60 ml/min/1.73 m2 vs. eGFR < 60 ml/min/1.73 m2, with a benefit favouring the lower eGFR, and for the presence vs absence of established CV disease at baseline, with benefit for those with CV disease at baseline.
- The liraglutide group showed a larger change in HbA1c value at 36 months than the placebo group (mean difference: −0.40% (95% CI: −0.45% to −0.34%)).
- The incidence of a composite outcome of renal or retinal microvascular events was lower in the liraglutide group than in the placebo group (HR: 0.84; 95% CI: 0.73 - 0.97; P = 0.02).
- Fewer nephropathy events were seen in the liraglutide group (1.5 vs. 1.9 events per 100 patient-years (PY) of observation; HR: 0.78; 95% CI: 0.67 - 0.92; P = 0.003).
- The incidence of retinopathy events was non-significantly higher in the liraglutide group than in the placebo group (0.6 vs. 0.5 events per 100 PY; HR: 1.15; 95% CI: 0.87 - 1.52; P = 0.33).
- Concerning safety outcomes, a non-significant increase of benign or malignant neoplasms were seen with liraglutide as compared with placebo, and a non-significantly lower incidence of acute pancreatitis.
- Adverse events leading to the permanent discontinuation of the trial regimen (mainly gastrointestinal events) were more common with liraglutide than with placebo.
Download Marso LEADER NEJM 2016_PACE.pptx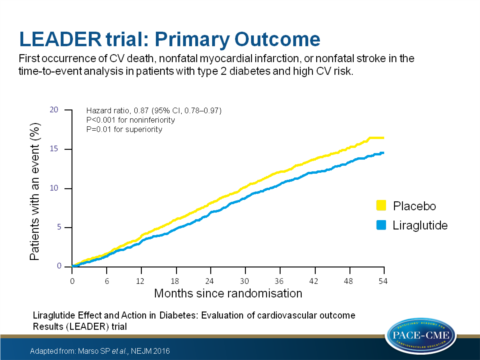
Conclusion
The LEADER outcome trial data show that in T2DM patients at high CV risk, liraglutide on top of standard therapy, was associated with lower rates of first occurrence of death from CV causes, nonfatal myocardial infarction or nonfatal stroke and death from any cause, compared with placebo in a time-to-event analysis.The LEADER Trial has been presented at ADA 2016, and was simultaneously published op NEJM
References
1. Holman RR, Sourij H, Califf RM. Cardiovascular outcome trials of glucoselowering drugs or strategies in type 2 diabetes. Lancet 2014; 383: 2008-17.
2. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. London: European Medicines Agency, 2012.
3. Guidance for industry: diabetes mellitus — evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. Silver Spring, MD: Department of Health and Human Services, 2008.
4. Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab 2016; 18: 203-16.
5. Du Q, Wang YJ, Yang S, et al. Liraglutide for the treatment of type 2 diabetes mellitus: a meta-analysis of randomized placebo-controlled trials. Adv Ther 2014; 31: 1182-95.
6. Robinson LE, Holt TA, Rees K, et al. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open 2013; 3.
