FFAR-1 agonists: a new option for type 2 diabetes mellitus?
09/03/2012
As FFAR 1 agonists increase insulin secretion glucose-dependently, those agents might improve glycaemic control without causing hypoglycaemia.
TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial.Literature - Burant CF et al, Lancet. 2012 Feb 24
Burant CF, Viswanathan P, Marcinak J, Cao C, Vakilynejad M, Xie B, Leifke E.
Lancet. 2012 Feb 24.
Abstract
Background: Activation of free fatty acid receptor 1 (FFAR1; also known as G-protein-coupled receptor 40) by fatty acids stimulated glucose-dependent β-cell insulin secretion in preclinical models. We aimed to assess whether selective pharmacological activation of this receptor by TAK-875 in patients with type 2 diabetes mellitus improved glycaemic control without hypoglycaemia risk.Methods: We undertook a phase 2, randomised, double-blind, and placebo-controlled and active-comparator-controlled trial in outpatients with type 2 diabetes who had not responded to diet or metformin treatment. Patients were randomly assigned equally to receive placebo, TAK-875 (6•25, 25, 50, 100, or 200 mg), or glimepiride (4 mg) once daily for 12 weeks. Patients and investigators were masked to treatment assignment. The primary outcome was change in haemoglobin A(1c) (HbA(1c)) from baseline. Analysis included all patients randomly assigned to treatment groups who received at least one dose of double-blind study drug. The trial is registered at ClinicalTrials.gov, NCT01007097.
Findings: 426 patients were randomly assigned to TAK-875 (n=303), placebo (n=61), and glimepiride (n=62). At week 12, significant least-squares mean reductions in HbA(1c) from baseline occurred in all TAK-875 (ranging from -1•12% [SE 0•113] with 50 mg to -0•65% [0•114] with 6•25 mg) and glimepiride (-1•05% [SE 0•111]) groups versus placebo (-0•13% [SE 0•115]; p value range 0•001 to <0•0001). Treatment-emergent hypoglycaemic events were similar in the TAK-875 and placebo groups (2% [n=7, all TAK-875 groups] vs 3% [n=2]); significantly higher rates were reported in the glimepiride group (19% [n=12]; p value range 0•010-0•002 vs all TAK-875 groups). Incidence of treatment-emergent adverse events was similar in the TAK-875 overall (49%; n=147, all TAK-875 groups) and placebo groups (48%, n=29) and was lower than in the glimepiride group (61%, n=38).
Interpretation: TAK-875 significantly improved glycaemic control in patients with type 2 diabetes with minimum risk of hypoglycaemia. The results show that activation of FFAR1 is a viable therapeutic target for treatment of type 2 diabetes.
The free fatty acid receptor 1 (FFAR1, or G-protein-coupled receptor 40, GPR40) is a cell-surface receptor with highest expression in β-cells of the pancreatic islets [1-4]. In the presence of raised glucose levels, activation of FFAR1 by fatty acids or synthetic ligands causes an increased secretion of insulin [5,6]. The cellular mechanism is different from those of that of the secretion initiators (sulphonylureas and meglitinides) and the incretin-based secretion potentiators (GLP-1 receptor agonists and DPP-4 inhibitor; fig. 1 [5]).
In type 2 diabetes mellitus, secretion of insulin from β-cells is inadequate. Drugs increasing that secretion are key in the treatment. As FFAR 1 agonists increase insulin secretion glucose-dependently, those agents might improve glycaemic control without causing hypoglycaemia [5,6].
TAK-875 is an oral, highly potent and selective FFAR1 agonist stimulating insulin secretion in a glucose-dependent manner in small clinical trials [7-9]. In this study the efficacy and safety of varying doses of TAK-875 were compared with placebo and glimepiride in patients with type 2 DM inadequately controlled by diet and exercise or treatment with metformin. Primary outcoume was change in HbA1c from baseline. At week 12, the percentage of patients reaching the ADA target of HbA1c< 7.0% was assessed in all groups.
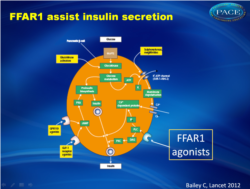 Stimulus-secretion coupling of pancreatic β cell to release insulin.cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; PLC; IP3, phospholipase C inositol trisphosphate; DAG, diacylglycerol |
Main results
For TAK-875 at week 12, significant reductions from baseline in HbA1c ranged from a least-squares mean of 0.65% (SE 0.114) at 6.25 mg to roughly 1.0% at doses of 50 mg and higher (1.12% [0.113] with 50 mg; figure 2A); a similar HbA1c reduction of 1.05% (SE 0.111) occurred in the glimepiride group. Mean change in HbA1c from baseline in the placebo group was –0.13% (SE 0.115). Only the decrease in the TAK-875 6.25 mg group was significantly smaller than that in the glimepiride group (p=0.012). At weeks 4, 8, and 12, significant (p≤0.0001), dose-dependent reductions from baseline in HbA1c occurred in TAK-875 dose groups 25–200 mg as well as in the glimepiride group compared with placebo (figure 2B). The reduction in HbA1c in TAK-875 dose groups 25–200 mg during the study period was similar to that obtained with glimepiride (fig. 2).Treatment-emergent hypoglycaemic events were similar in the TAK-875 and placebo groups (2%, n=7, all TAK-875 groups and 3%, n=2, respectively), whereas significantly higher rates were reported in the glimepiride group (19%, n=12)
Conclusion
Between 33% and 48% of patients reached the ADA target of HbA1c < 7% at week 12 at doses of TAK-875 25 mg and higher, similar to those reported in patients treated with glimepiride. The low risk of hypoglycaemia after treatment with TAK-875 suggests that targeting FFAR1 in patients with t2DM might have a therapeutic advantage. Because of the short study period, small sample size and multiple gropu comparisons, longert-term larger studies are necessary to elucidate the effects.Editorial comment [5]
FFAR 1 agonists are an interesting therapeutic option in treating type 2 diabetes. Selective FFAR1 agonists might be capable of restricted initiation of insulin secretion, and will mainly potentiate nutrient-induced insulin secretion, which will favour enhanced prandial insulin secretion and reduce the risk of interprandial hypoglycaemia [11,12]. On the journey to approval of a new class of treatment for type 2 diabetes, many questions will be asked of the FFAR1 agonists. Can they unlock the secretion-shy β cells, provide durable efficacy, and avoid off -target safety issues? We travel hopefully.References
1. Cornish J, MacGibbon A, Lin JM, et al. Modulation of osteoclastogenesis by fatty acids. Endocrinology 2008; 149: 5688–95.2. Hidalgo MA, Nahuelpan C, Manosalva C, et al. Oleic acid induces intracellular calcium mobilization, MAPK phosphorylation, superoxide production and granule release in bovine neutrophils. Biochem Biophys Res Commun 2011; 409: 280–86.
3. Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 2008; 57: 2280–87.
4. Briscoe CP, Tadayyon M, Andrews JL, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 2003; 278: 11303–11.
5. Bailey CJ. Could FFAR1 assist insulin secretion in type 2 diabetes? Lancet. 2012 Feb 24. [Epub ahead of print]
6. Nagasumi K, Esaki R, Iwachidow K, et al. Overexpression of GPR40 in pancreatic beta-cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice. Diabetes 2009; 58: 1067–76.
7. Itoh Y, Hinuma S. GPR40, a free fatty acid receptor on pancreatic beta cells, regulates insulin secretion. Hepatol Res 2005; 33: 171–73.
