GAUSS: PCSK9 antibody reduces LDL in statin-intolerant patients
04/01/2013
JAMA, Dec 2012 . Patients at CV risk who are unable to tolerate effective doses of statins experienced reductions in LDL levels of up to 51% with AMG 145, an investigational, anti-PCSK9 antibody.
Effect of a Monoclonal Antibody to PCSK9 on Low-Density Lipoprotein Cholesterol Levels in Statin-Intolerant Patients: The GAUSS Randomized Trial.Literature -
Sullivan D, Olsson AG, Scott R, et al.
JAMA. 2012;308:2497-506. doi: 10.1001/jama.2012.25790.
Reduction of LDL-C is the cornerstone in CV risk reduction [1-3], with statins being the most effective drugs for reducing LDL-C [4]. However, approximately 10-20% of patients cannot tolerate (high-dose) statins, mainly due to muscle-related side effects [5]. Ezetimibe, a cholesterol absorption inhibitor, is an effective and frequently used alternative, mostly used in combination with statins [6], but more effective LDL-C lowering therapies are needed for statin-intolerant patients.
The GAUSS study was initiated to test the safety, tolerability and efficacy of subcutaneous AMG145, which binds to PCSK9 in the circulation and blocks its interaction with the LDL receptor and thus increases cholesterol removal from the blood stream [7,8]. Its use was compared with ezetimibe in statin-intolerant patients at high and moderate CV risk.
The 12-week, randomized, double blind, controlled, dose-ranging study was conducted between July 2011 and May 2012 and enrolled 236 patients. Patients were randomly assigned to one of five groups: AMG145 280 mg, 350 mg or 420 mg alone; AMG 145 420 mg plus 10 mg ezetimibe; or 10 mg ezetimibe plus placebo. AMG 145 and placebo were administered subcutaneously every 4 weeks.
1. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421.
2. Reiner Z, Catapano AL, De Backer G, et al; European Association for Cardiovascular Prevention & Rehabilitation; ESC Committee for Practice Guidelines (CPG) 2008-2010 and 2010-2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC)and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769-1818.
3. McPherson R, Frohlich J, Fodor G, Genest J; Canadian Cardiovascular Society. Canadian Cardiovascular Society position statement: recommendations for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease. Can J Cardiol. 2006;22(11):913-927.
4. Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267-1278.
5. Bruckert E, Hayem G, Dejager S, Yau C, Be´gaud B. Mild to moderate muscular symptoms with highdosage statin therapy in hyperlipidemic patients: the PRIMO study. Cardiovasc Drugs Ther. 2005;19(6):403-414.
6. Ballantyne CM, Weiss R, Moccetti T, et al; EXPLORER Study Investigators. Efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the EXPLORER study). Am J Cardiol. 2007;99(5):673-680.
7. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264-1272.
8. Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res. 2009;50(suppl):S172-S177.
Context
An estimated 10% to 20% of patients cannot tolerate statins or adequate doses to achieve treatment goals. Plasma proprotein convertase subtilisin/kexin type 9 (PCSK9) binds to low-density lipoprotein (LDL) receptors, promoting their degradation and increasing LDL cholesterol levels. In phase 1 studies, a human monoclonal antibody to PCSK9, AMG145, was well tolerated and reduced LDL cholesterol levels.
Objective
To assess the efficacy and tolerability of AMG145 in patients with statin intolerance due to muscle-related side effects.
Design, setting, and patients
A 12-week, randomized, double-blind, placebo- and ezetimibe-controlled, dose-ranging study conducted between July 2011 and May 2012 in statin-intolerant adult patients at 33 international sites.
Intervention
Patients were randomized equally to 1 of 5 groups: AMG145 alone at doses of 280 mg, 350 mg, or 420 mg; AMG145 at 420 mg plus 10 mg of ezetimibe; or 10 mg of ezetimibe plus placebo. AMG145 or placebo was administered subcutaneously every 4 weeks.
Main outcome measures
The primary end point was percentage change from baseline to week 12 in ultracentrifugation-measured LDL cholesterol. Other end points included measures of safety and tolerability of different doses of AMG145 and AMG145 plus ezetimibe.
Results
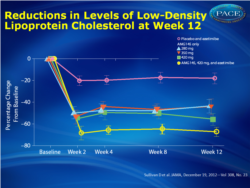
Of 236 patients screened, 160 were randomized (mean age, 62 years; 64% female; mean baseline LDL cholesterol, 193 mg/dL); all patients had intolerance to 1 or more statins because of muscle-related events. At week 12, mean changes in LDL cholesterol levels were -67 mg/dL (-41%; 95% CI, -49% to -33%) for the AMG145, 280-mg, group; -70 mg/dL (-43%; 95% CI, -51% to -35%) for the 350-mg group; -91 mg/dL (-51%; 95% CI, -59% to -43%) for the 420-mg group; and -110 mg/dL (-63%; 95% CI, -71% to -55%) for the 420-mg/ezetimibe group compared with -14 mg/dL (-15%; 95% CI, -23% to -7.0%) for the placebo/ezetimibe group (P < .001). Four serious adverse events were reported with AMG145 (coronary artery disease, acute pancreatitis, hip fracture, syncope). Myalgia was the most common treatment-emergent adverse event during the study, occurring in 5 patients (15.6%) in the 280-mg group (n = 32); 1 patient (3.2%) in the 350-mg group (n = 31), 1 patient (3.1%) in the 420-mg group (n = 32), 6 patients (20.0%) receiving 420-mg AMG145/ezetimibe, and 1 patient (3.1%) receiving placebo/ezetimibe.
Conclusion
In this phase 2 study in statin-intolerant patients, subcutaneous administration of a monoclonal antibody to PCSK9 significantly reduced LDL cholesterol levels and was associated with short-term tolerability.
JAMA. 2012;308:2497-506. doi: 10.1001/jama.2012.25790.
Background
Reduction of LDL-C is the cornerstone in CV risk reduction [1-3], with statins being the most effective drugs for reducing LDL-C [4]. However, approximately 10-20% of patients cannot tolerate (high-dose) statins, mainly due to muscle-related side effects [5]. Ezetimibe, a cholesterol absorption inhibitor, is an effective and frequently used alternative, mostly used in combination with statins [6], but more effective LDL-C lowering therapies are needed for statin-intolerant patients.The GAUSS study was initiated to test the safety, tolerability and efficacy of subcutaneous AMG145, which binds to PCSK9 in the circulation and blocks its interaction with the LDL receptor and thus increases cholesterol removal from the blood stream [7,8]. Its use was compared with ezetimibe in statin-intolerant patients at high and moderate CV risk.
The 12-week, randomized, double blind, controlled, dose-ranging study was conducted between July 2011 and May 2012 and enrolled 236 patients. Patients were randomly assigned to one of five groups: AMG145 280 mg, 350 mg or 420 mg alone; AMG 145 420 mg plus 10 mg ezetimibe; or 10 mg ezetimibe plus placebo. AMG 145 and placebo were administered subcutaneously every 4 weeks.
Main results
- At 12 weeks, mean LDL decreased 41% for patients assigned AMG 145 280 mg; 43% for AMG 145 350 mg; 51% for AMG 145 420 mg; 63% for AMG 145 420 mg plus ezetimibe; and 15% for placebo plus ezetimibe (P<.001 vs. placebo and ezetimibe)
- Sixty-one percent of patients assigned AMG 145 420 mg achieved an LDL goal of <100 mg/dL and up to 29% reached <70 mg/dL. When combined with ezetimibe, 90% of patients reached the goal of <100 mg/dL and 62% reached <70 mg/dL.
- Data also demonstrated benefits in total cholesterol, non-HDL, lipoprotein(a), apolipoprotein B, and the ratios of total cholesterol/HDL and ApoB/ApoA1. AMG 145 alone or with ezetimibe increased HDL modestly, from 6% to 12%, compared with a 1% decrease with ezetimibe alone (P<.001). Small, nonsignificant reductions in triglycerides and VLDL were observed with AMG 145 compared with ezetimibe alone. Free PCSK9 levels declined by 48% from baseline to 12 weeks with AMG 145 and by 2% with ezetimibe alone.
- Myalgia was the most common treatment-emergent adverse event during the study. Other adverse events included nasopharyngitis, nausea and fatigue. Overall, the drug was well tolerated and efficacious, with or without ezetimibe.
Conclusion
In patients with prior statin intolerance, treatment with AMG145, achieved significant reductions in LDL-C and was associated with short-term tolerability.
References
1. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421.2. Reiner Z, Catapano AL, De Backer G, et al; European Association for Cardiovascular Prevention & Rehabilitation; ESC Committee for Practice Guidelines (CPG) 2008-2010 and 2010-2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC)and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769-1818.
3. McPherson R, Frohlich J, Fodor G, Genest J; Canadian Cardiovascular Society. Canadian Cardiovascular Society position statement: recommendations for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease. Can J Cardiol. 2006;22(11):913-927.
4. Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267-1278.
5. Bruckert E, Hayem G, Dejager S, Yau C, Be´gaud B. Mild to moderate muscular symptoms with highdosage statin therapy in hyperlipidemic patients: the PRIMO study. Cardiovasc Drugs Ther. 2005;19(6):403-414.
6. Ballantyne CM, Weiss R, Moccetti T, et al; EXPLORER Study Investigators. Efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the EXPLORER study). Am J Cardiol. 2007;99(5):673-680.
7. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264-1272.
8. Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res. 2009;50(suppl):S172-S177.
Abstract
ContextAn estimated 10% to 20% of patients cannot tolerate statins or adequate doses to achieve treatment goals. Plasma proprotein convertase subtilisin/kexin type 9 (PCSK9) binds to low-density lipoprotein (LDL) receptors, promoting their degradation and increasing LDL cholesterol levels. In phase 1 studies, a human monoclonal antibody to PCSK9, AMG145, was well tolerated and reduced LDL cholesterol levels.
Objective
To assess the efficacy and tolerability of AMG145 in patients with statin intolerance due to muscle-related side effects.
Design, setting, and patients
A 12-week, randomized, double-blind, placebo- and ezetimibe-controlled, dose-ranging study conducted between July 2011 and May 2012 in statin-intolerant adult patients at 33 international sites.
Intervention
Patients were randomized equally to 1 of 5 groups: AMG145 alone at doses of 280 mg, 350 mg, or 420 mg; AMG145 at 420 mg plus 10 mg of ezetimibe; or 10 mg of ezetimibe plus placebo. AMG145 or placebo was administered subcutaneously every 4 weeks.
Main outcome measures
The primary end point was percentage change from baseline to week 12 in ultracentrifugation-measured LDL cholesterol. Other end points included measures of safety and tolerability of different doses of AMG145 and AMG145 plus ezetimibe.
Results
Of 236 patients screened, 160 were randomized (mean age, 62 years; 64% female; mean baseline LDL cholesterol, 193 mg/dL); all patients had intolerance to 1 or more statins because of muscle-related events. At week 12, mean changes in LDL cholesterol levels were -67 mg/dL (-41%; 95% CI, -49% to -33%) for the AMG145, 280-mg, group; -70 mg/dL (-43%; 95% CI, -51% to -35%) for the 350-mg group; -91 mg/dL (-51%; 95% CI, -59% to -43%) for the 420-mg group; and -110 mg/dL (-63%; 95% CI, -71% to -55%) for the 420-mg/ezetimibe group compared with -14 mg/dL (-15%; 95% CI, -23% to -7.0%) for the placebo/ezetimibe group (P < .001). Four serious adverse events were reported with AMG145 (coronary artery disease, acute pancreatitis, hip fracture, syncope). Myalgia was the most common treatment-emergent adverse event during the study, occurring in 5 patients (15.6%) in the 280-mg group (n = 32); 1 patient (3.2%) in the 350-mg group (n = 31), 1 patient (3.1%) in the 420-mg group (n = 32), 6 patients (20.0%) receiving 420-mg AMG145/ezetimibe, and 1 patient (3.1%) receiving placebo/ezetimibe.
Conclusion
In this phase 2 study in statin-intolerant patients, subcutaneous administration of a monoclonal antibody to PCSK9 significantly reduced LDL cholesterol levels and was associated with short-term tolerability.