Genetic predisposition to high LDL-c associated with aortic valve disease
Mendelian randomisation approach points at causal role for increased LDL-c in the development of aortic stenosis and presence of aortic valve calcium.
Association of Low-Density Lipoprotein Cholesterol-Related Genetic Variants With Aortic Valve Calcium and Incident Aortic StenosisLiterature - Smith JG et al., JAMA 2014
Smith JG, Luk K, Schulz CA, et al.; for the Cohorts for Heart and Aging Research in Genetic Epidemiology (CHARGE) Extracoronary Calcium Working Group.
JAMA. 2014 Oct 26. doi: 10.1001/jama.2014.13959. [Epub ahead of print]
Background
It is largely unknown what causes aortic valve disease, and no medical treatment is available to stop or retard disease progression. An association between a common variant in the LPA gene, via elevated plasma lipoprotein(a) (Lp[a]), and aortic valve disease has recently been shown [1]. It is, however, unclear whether other plasma lipids are causally associated with the development of aortic valve disease.While epidemiological studies have shown that LDL-c is an important risk factor for aortic valve disease, randomised trials of LDL-c lowering therapy in patients with advanced aortic stenosis did not demonstrate an effect on disease progression [2-4]. LDL-c may, however, still play a causal role in earlier stages of aortic valve disease.
‘Mendelian randomisation’ is an approach that benefits from the random allocation of genetic information that occurs at conception, to identify potentially causal biomarkers [1,5]. Genetic risk scores (GRSs) for lipids, as a summary score of multiple genetic variants, are strongly associated with actual lipid levels in both children and adults [6,7], suggesting that a higher GRS confers life-long exposure to higher lipid levels.
This study aimed to establish, via a Mendelian randomisation approach, whether genetic contributions to elevations in LDL-c and other lipids are associated with early subclinical aortic valve disease (3 CHARGE cohorts) and incident clinical aortic stenosis (Malmö Diet and Cancer Study (MDCS), over 15 years of follow-up).
Main results
- Baseline LDL-c, but not HDL-c or triglyceride (TG) levels were significantly associated with incident aortic stenosis (HR per mmol/L: 1.28, 95%CI: 1.04-1.57, P=0.02). Incidence of aortic stenosis was 1.3% vs. 2.4% in the lowest and highest LDL-c quartiles, respectively.
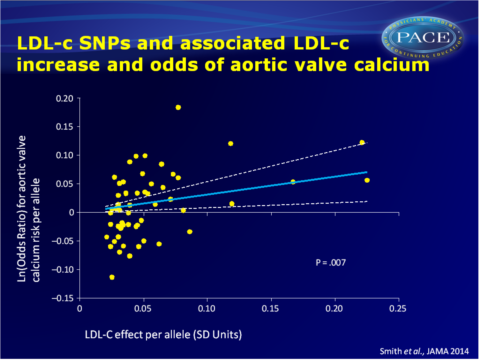
- In the CHARGE cohorts, the LDL-c GRS (but not the HDL-c and TG GRSs) was significantly associated with presence of aortic valve calcium (OR per GRS increment: 1.38, 95%CI: 1.09-1.74, P=0.007). After exclusion of SNPs that are also associated with other lipid traits, a ‘specific’ LDL-c GRS remained associated with presence of aortic valve calcium (OR per GRS increment: 1.39, 95%CI: 1.04-1.86, P=0.03).
- In MDCS participants, of whom direct genotype-phenotype data were available, the LDL-c GRS (but not the HDL-c and TG GRSs) was significantly associated with increased aortic stenosis incidence (HR per GRS increment in LDL-c: 2.78, 95%CI: 1.22-6.37, P=0.02, 1.9% vs. 2.6% in the lowest and highest GRS quartiles).
- In MDCS, the ‘specific’ LDL-c GRS was significantly associated with incident aortic stenosis (HR per GRS increment: 3.85, 95%CI: 1.37-10.79, P=0.009).
- A multivariable analysis suggested a strong linear association between the magnitude of change in LDL-c and the increase in aortic valve calcium presence, which did not substantially change after adjusting for HDL and TG.
Download Smith JAMA 2014 pace.pptx
Conclusion
Genetic elevation of LDL-c, as determined by a GRS, was found to be associated with presence of aortic valve calcium and incident aortic stenosis. No such associations were seen between HDL-c or TG GRS and aortic stenosis or valve calcium, suggesting that a mechanism specifically attributable to LDL-c is responsible for the association with aortic valve disease. It should therefore be investigated whether earlier intervention to reduce LDL-c in genetically predisposed individuals can prevent aortic valve disease.Find this article on Pubmed
References
1. Thanassoulis G, Campbell CY, Owens DS, et al; CHARGE Extracoronary CalciumWorking Group. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368(6):503-512.
2. Cowell SJ, Newby DE, Prescott RJ, et al; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352(23):2389- 2397.
3. Rossebø AB, Pedersen TR, Boman K, et al; SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359(13):1343-1356.
4. Chan KL, Teo K, Dumesnil JG, et al.; ASTRONOMER Investigators. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121(2):306-314.
5. Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Et al. Genetically elevated lipoprotein(a) and increased risk ofmyocardial infarction. JAMA. 2009;301(22):2331-2339.
6. Tikkanen E, Tuovinen T, Widén E, et al. Association of known loci with lipid levels among children and prediction of dyslipidemia in adults. Circ Cardiovasc Genet. 2011;4(6):673-680.
7. Do R,Willer CJ, Schmidt EM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45 (11):1345-1352.
