Greater reduction in LDL-C with PCSK9-inhibitor alirocumab than with ezetimibe in inadequately controlled hypercholesterolaemia
Results of the ODYSSEY COMBO II trial show that alirocumab produced significantly greater reductions in LDL-C compared to ezetimibe with a comparable safety profile in high cardiovascular risk patients with inadequately controlled LDL-C on maximally tolerated doses of potent statins.
Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial.Literature - Cannon et al., Eur Heart J 2015
Eur Heart J. 2015 Feb 16, doi:10.1093/eurheartj/ehv028
Background
The reduction of low-density lipoprotein cholesterol (LDL-C) is the primary target to reduce cardiac events [1-3]. LDL-C reduction with statins lowers the risk of CHD events and all-cause mortality [4]. A substantial proportion of high-risk hypercholesterolaemic patients do not achieve adequate LDL-C reductions, in spite of intensive statin therapy [5,6]. Evidence is needed on new lipid-modifying agents, on top of statin therapy, regarding the event reduction in cardiovascular disease [7]. These therapies include fully human monoclonal antibodies against proprotein convertase subtilisin/kexin 9 (PCSK9), such as alirocumab and evolocumab. Alirocumab reduces LDL-C concentrations by 40–70% in combination with other lipid-lowering therapies (LLT) or as monotherapy. The COMBO II study compares the efficacy and safety of alirocumab versus the non- statin agent ezetimibe as add-on therapy to maximally tolerated statin therapy in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia. The COMBO II is an ongoing double-blind, double-dummy, active-controlled, parallel-group, 104-week study that is conducted at 126 sites. Patients (n=720) were randomized to either 75 mg subcutaneous alirocumab every 2weeks (plus oral placebo) or 10 mg daily oral ezetimibe (plus subcutaneous placebo).
Main results
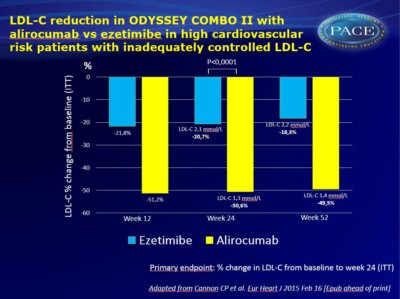
- After 24 weeks, a reduction in LDL-C of 50.6% was seen with alirocumab, and 20.7% with ezetimibe, both in addition to maximum tolerated statin treatment (LS mean difference (SE) vs. ezetimibe: -29.8% (2.3) P <0.0001).
- 77.0% of the patients achieved LDL-C target levels <1.8 mmol/L, compared to 45.6% of the ezetimibe-treated patients (P< 0.0001).
- 18% of patients randomized to alirocumab needed dose adjustment based on their LDL-C levels at week 12.
- The LDL-C decrease achieved with alirocumab was stable during the 52-week follow-up.
- These results were consistent across various patient subgroups.
- Typical statin-related adverse events were equally seen in the treatment arms. Small numerical differences in relevant side effects were observed, like more verified CV events (4.8% vs. 3.7% in alirocumab vs. ezetimibe), more injection-site reactions (2.5% vs. 0.8% with placebo injection), less neurocognitive disorders (0.8 % vs. 1.2%), more often ALT> 3x ULN (1.7% vs. 0.4%) and creatine kinase> 3x ULN (2.8% vs. 2.5%). The alirocumab injections were generally well tolerated and compliance with self-injections was good.
Conclusions
Adding alirocumab to a treatment regimen with maximally tolerated statins provided safe and substantial LDL-C reductions and more patients with hypercholesterolaemia can achieve LDL-C goals than by adding ezetimibe. These results are in line with the previous suggestion that maximum LDL-C response to a PCSK9 inhibitor is greater with combination therapy than with monotherapy. The COMBO II study is ongoing and will continue up to 104 weeks follow-up to maximize the available safety data and generate information on the durability of alirocumab lipid-lowering effects [8].Find this article on eurheartj.oxfordjournals.org
References
1. Cholesterol Treatment Trialists (CTT) Collaboration, Baigent C, Blackwell L,
Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–1681.
2. Perk J, DeBacker G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2012;33:1635–1701.
3. Reiner Z, Catapano AL,DeBacker G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769–1818.
4. Cholesterol Treatment Trialists C, Mihaylova B, Emberson J, et al,. The effects of lowering
LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581–590.
5. Banegas JR, Lopez-Garcia E, Dallongeville J, et al. Achievement of treatment goals for primary prevention of cardiovascular disease in clinical practice across Europe: the EURIKA study. Eur Heart J 2011;32:2143–2152.
6. Hermans MP, Castro Cabezas M, Strandberg T, et al. Centralized Pan-European survey on the under-treatment of hypercholesterolaemia (CEPHEUS): overall findings from eight countries. Curr Med Res Opin 2010;26:445–454.
7. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(Suppl. 2):S1–S45.
8. Colhoun HM, Robinson JG, Farnier M, et al. PCSK9 monoclonal antibody, in high cardiovascular risk patients with poorly controlled hypercholesterolemia on maximally tolerated doses of statins: rationale and design of the ODYSSEY COMBO I and II trials. BMC Cardiovasc Disord 2014;14:121.
