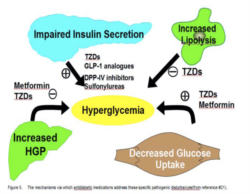
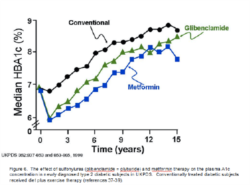
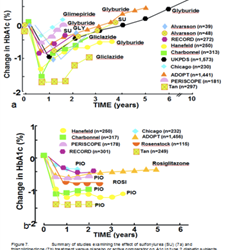
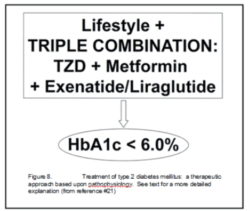
Holistic Approach to Glycemic control
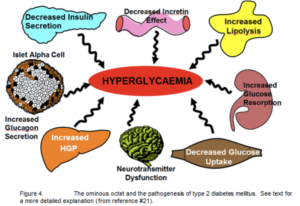
Introduction Ten years ago, our concept of the pathogenesis of type 2 diabetes was quite simple. We recognised that there was underlying moderate-severe insulin resistance affecting muscle and the liver, and this, coupled with beta cell failure, formed the classical triumvirate (1). The insulin resistance in muscle primarily was responsible for the excessive postprandially rise in plasma glucose concentration, while insulin resistance in the liver, in combination with accelerated gluconeogenesis, resulted in an excessive rate of hepatic glucose production which led to an increase in the fasting plasma glucose concentration (2-4).
HOLISTIC APPROACH TO GLYCEMIC CONTROLNews - Mar. 30, 2011
Ralph A. DeFronzo, M.D., Professor of Medicine, Chief, Diabetes Division, University of Texas Health Science Center, San Antonio, TX
Introduction
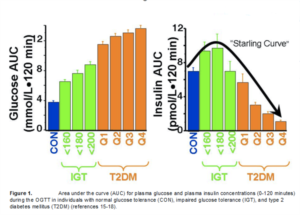
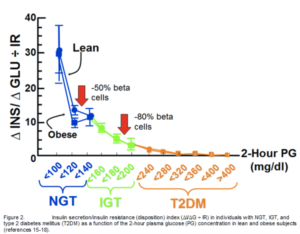
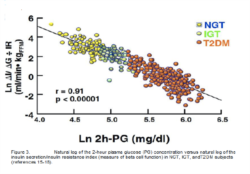
Ten years ago, our concept of the pathogenesis of type 2 diabetes was quite simple. We recognised that there was underlying moderate-severe insulin resistance affecting muscle and the liver, and this, coupled with beta cell failure, formed the classical triumvirate (1). The insulin resistance in muscle primarily was responsible for the excessive postprandially rise in plasma glucose concentration, while insulin resistance in the liver, in combination with accelerated gluconeogenesis, resulted in an excessive rate of hepatic glucose production which led to an increase in the fasting plasma glucose concentration (2-4). In response to the insulin resistance, the beta cell initially is able to compensate, by appropriately increasing its secretion of insulin to maintain normal glucose homeostasis (1,5,6). However, with time declining beta cell function results first in postprandial hyperglycaemia and subsequently a rise in the fasting plasma glucose concentration and ultimately the onset of frank diabetes (5,7-9). The relative contributions of beta cell failure and insulin resistance to the diabetic state vary between different ethnic populations (10). However diabetes does not become manifest until beta cell failure leads to a failure to compensate for the insulin resistance(1,11).
6. Diamond MP, Thornton K, Connolly-DiamonM, Sherwin RS, DeFronzo RA. Reciprocal variations in insulin-stimulated glucose uptakeand pancreatic insulin secretion in women witnormal glucose tolerance. J Soc Gynecol Investig 1995;2:708-15. 7. Lillioja S, Mott DM, Spraul M, Ferraro R, JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 1993;329:1988-92.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 2004;88:787-835. Bergman RN, Finegood DT, Kahn SE. The evolution of beta-cell dysfunction and insulin resistance in type 2 diabetes. Eur J Clin Invest 2002;32 Suppl 3:35-45. Abdul-Ghani M MM, Sabbah M, Jenkinson C, Richardson DK, DeFronzo RA. The relative contribution of insulin resistance and beta cell failure to the transitiion from normal to impairedglucose tolerance varies in different ethnic groups. Diabete Metab Synd 2007;1:105-12. Jallut D, Golay A, Munger R, Frascarolo P Schutz Y, Jequier E, Felber JP. Impaired glucose tolerance and diabetes in obesity: a 6-year follow-up study of glucose metabolism.Metabolism 1990;39:1068-75. Gulli G, Ferrannini E, Stern M, Haffner S, DeFronzo RA. The metabolic profile of NIDDM is fully established in glucose-tolerant offspring of two Mexican-American NIDDM parents. Diabetes 1992;41:1575-86. 13.Pratipanawatr W, Pratipanawatr T, Cusi K, Berria R, Adams JM, Jenkinson CP, MaezonK, DeFronzo RA, Mandarino LJ: Skeletal muscle insulin resistance in normoglycemic subjects with a strong family history of type 2 diabetes is associated with decreased insulin-stimulated insulin receptor substrate-1 tyrosinephosphorylation. Diabetes 2001; 50:2572-8. Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI: ed Reduced mitochondrial density and increasIRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 2005; 115:3587-93. Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA: Insulin secrand action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 200 55:1430-5. 16. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA: Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) studDiabetologia 2004; 47:31-9.