Hypercholesterolemia: Clinical and MOA update on ezetimibe
05/10/2012
Ezetimibe inhibits intestinal cholesterol absorption. Its role in hypercholesterolemia has been studied, both in monotherapy and in combination with statins. New trial results are awaited to determine the possible use of ezetimibe in clinical practice.
Ezetimibe therapy: mechanism of action and clinical update.Literature -
Phan BA, Dayspring TD, Toth PP.
Vasc Health Risk Manag. 2012;8:415-27.
Background
There is a clear association between elevated serum cholesterol levels and cardiovascular risk [1,2]. Treatment with statins effectively lowers LDL-C levels and reduces major cardiovascular events [3-7]. More aggressive lowering of LDL-C may increase cardiovascular benefit [8-10], especially in high-risk patients with multiple risk factors [11]. To achieve optimal LDL-C levels, statin therapy alone might not be sufficient. In these patients, combination with other cholesterol-lowering agents is warranted, such as the cholesterol absorption inhibitor ezetimibe.The mechanism of action, lipid effects, and safety of ezetimibe treatment are described as well as outcome trials that may impact its use in clinical practice.
Summary
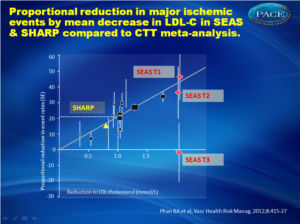
Ezetimibe inhibits intestinal cholesterol absorption by selectively blocking the Niemann-Pick C1-like protein (NPC1L1) in the jejunal brush border, integral to the uptake of intestinal lumen micelles into the enterocyte [12-15]. It is, either in monotherapy or in combination with statins, effective in lowering cholesterol in several populations, such as insulin resistance, FH and sitosterolemia.Both the SANDS and VYCTOR trial reported atherosclerosis regression [16-18]. The clinical efficacy of ezetimibe treatment was evaluated in the SEAS study [19] and in the SHARP trial [20], both using ezetimibe plus simvastatin (figure 1).
However, the ENHANCE [21] and ARBITER-6 trial [22] reported negative outcomes, although both trials were methodologically limited in their ability to evaluate the benefit of ezetimibe.
Therefore, the forthcoming results from the IMPROVE-IT trial [23] are highly anticipated. These results may better guide the use of ezetimibe in very high-risk CHD populations.
Conclusion
Yet, ezetimibe is a viable adjunct to statin therapy in the treatment of hypercholesterolemia. Forthcoming results are awaited to better judge its effect in clinical practice.
References
1. Kannel WB, Castelli WP, Gordon T. Cholesterol in the prediction of atherosclerotic disease. An Intern Med. 1979;90:85–91.2. Keys A, Menotti A, Aravanis C, et al. The seven countries study: 2,289 deaths in 15 years. Prev Med. 1984;13:141–154.
3. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22.
4. [No authors listed]. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389.
5. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009.
6. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333: 1301–1307.
7. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA. 1998;279: 1615–1622.
8. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435.
9. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
10. Cannon CP, Steinberg BA, Murphy SA, Mega JL Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–445.
11. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–732.
12. Bays H. Ezetimibe. Expert Opin Investig Drugs. 2002;11:1587–1604.
13. Altmann SW, Davis HR Jr, Zhu LJ, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204.
14. Rosenblum SB, Huynh T, Afonso A, et al. Discovery of 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4-hydroxyphenyl)-2-azetidinone (SCH 58235): a designed, potent, orally active inhibitor of cholesterol absorption. J Med Chem. 1998;41: 973–980.
15. Davis HR Jr, Tershakovec AM, Tomassini JE, Musliner T. Intestinal sterol transporters and cholesterol absorption inhibition. Curr Opin Lipidol. 2011;22:467–478.
16. Howard BV, Roman MJ, Devereux RB, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: the SANDS randomized trial. JAMA. 2008;299:1678–1689.
17. Fleg JL, Mete M, Howard BV, et al. Effect of statins alone versus statins plus ezetimibe on carotid atherosclerosis in type 2 diabetes: the SANDS (Stop Atherosclerosis in Native Diabetics Study) trial. J Am Coll Cardiol. 2008;52:2198–2205.
18. Meaney A, Ceballos G, Asbun J, et al. The VYtorin on Carotid intima-media thickness and overall arterial rigidity (VYCTOR) study. J Clin Pharmacol. 2009;49:838–847.
19. Rossebø AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359: 1343–1356.
20. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192.
21. Kastelein JJP, Akdim F, Stroes ESG, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. New Engl J Med. 2008;358: 1431–1443.
22. Taylor AJ, Villines TC, Stanek EJ, et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009;361: 2113–2122.
23. Cannon CP, Giugliano RP, Blazing MA, et al. Rationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial): comparison of ezetimbe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes in patients with acute coronary syndromes. Am Heart J. 2008;156:826–832.