Icosapent ethyl reduces coronary revascularization
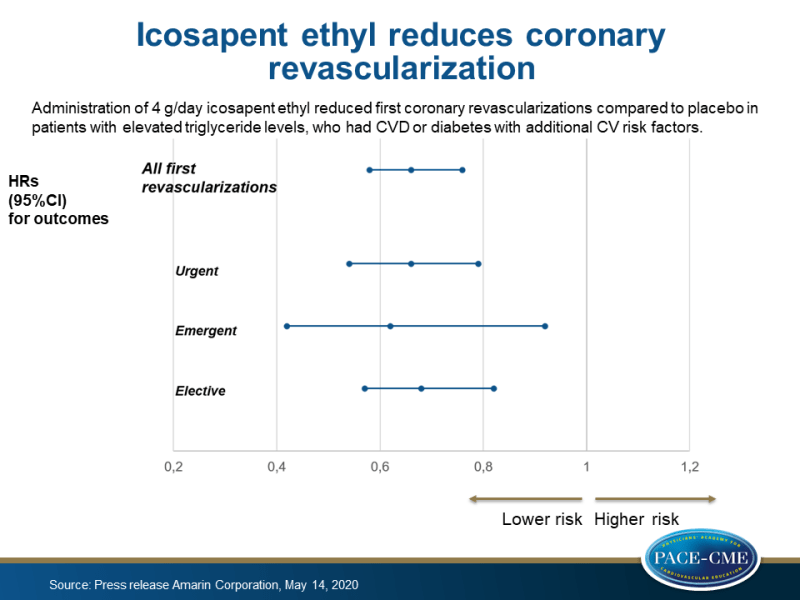
Administration of 4 g/day icosapent ethyl reduced first coronary revascularizations by 34% compared to placebo in patients with elevated triglyceride levels, who had CVD or diabetes with additional CV risk factors.
News - May 20, 2020First and total (first and subsequent) coronary revascularization, including coronary stenting and cardiac bypass surgery, were significantly reduced by 34% and 36%, respectively with administration of 4/g day icosapent ethyl compared to placebo (both P<0.0001), shown in prespecified tertiary endpoint analyses of the REDUCE-IT trial.
These analyses from the REDUCE-IT trial included several types of coronary revascularization events in statin-treated patients with elevated triglyceride levels (135-499 mg/dL), who had CVD or diabetes with additional CV risk factors. Prespecified tertiary endpoints of urgent, emergent and elective revascularizations were reduced by 34% (P<0.0001), 38% (P=0.02), and 32% (P<0.0001), respectively, in those who received icosapent ethyl compared to placebo.
Post-hoc analyses showed that icosapent ethyl reduced percutaneous coronary intervention (PCI) by 32% (P<0.0001) and coronary artery bypass grafting (CABG) by 39% (P=0.0005) compared to placebo.
These findings were presented at the Society for Cardiovascular Angiography & Interventions 2020 Scientific Sessions by Benjamin Peterson, MD, Brigham and Women’s Hospital and Harvard Medical School.
REDUCE-IT was a global, CV outcomes trial that evaluated the effect of icosapent ethyl in patients on statin therapy who had elevated triglycerides between 135-499 mg/dL and established CVD or diabetes and at least one CV risk factor. 8179 Patients were enrolled at >400 clinical sites in 11 countries. Primary results were published in the NEJM in November 2018. Administration of icosapent ethyl resulted in reduction of ischemic events .
Icosapent ethyl capsules are comprised solely of the active ingredient icosapent ethyl, a unique from of eicosapentaenoic acid. It was initially approved for use as an adjunct therapy to diet to reduce triglyceride levels in adults with severe (≥500 mg/dL) hypertriglyceridemia. In December 2019, the new CV risk indication for icosapent ethyl was approved by the FDA.