Inhibition of IL-1β lowers inflammatory response in type 2 diabetes
A single dose of human monoclonal antibody against IL-1β (canakinumab) gives a potent reduction of hsCRP levels up to 12 weeks.
Pharmacokinetic and Pharmacodynamic Characteristics of Single-Dose Canakinumab in Patients With Type 2 Diabetes Mellitus.Literature - Noe et al., Clin Ther. 2014 - Clin Ther. 2014 Sep 17
Noe A, Howard C, Thuren T et al.,
Clin Ther. 2014 Sep 17. doi: 10.1016/j.clinthera.2014.08.004. [Epub ahead of print]
Background
Although most clinical studies use morbidity and all-cause mortality as parameters to assess cardiovascular (CV) outcomes in patients with type 2 diabetes mellitus (T2DM), surrogate biomarkers such as high-sensitivity C-reactive protein (hsCRP), interleukin (IL)-6 and fibrinogen may provide a clearer understanding of early inflammatory changes in response to treatment. Although hsCRP is not a validated biomarker yet, different lines of evidence suggest that an increase in hsCRP levels are associated with an increase in CV risk [1-4].Canakinumab is a human monoclonal antibody directed against IL-1β, which prevents binding of IL-1β to the IL-1 receptors. Canakinumab targets the IL-1β-dependent inflammation pathway, thereby lowering hsCRP levels and those of other inflammatory CV biomarkers [5]. IL-1β-blockade has been shown to improve β-cell function and to prevent or delay the destruction of β-cells and worsening of diabetes in patients with T2DM [6]. Little is known about the use of canakinumab in patients with T2DM.
The efficacy and tolerability of canakinumab was therefore tested in patients with T2DM; this article describes the pharmacokinetic and pharmacodynamic data, including hsCRP levels, and change in HbA1c and tolerability data. 222 patients with T2DM completed this multicentre, randomised, double-blind, single-dose placebo-controlled study. Patients were on a stable daily dose of metformin.
Main results
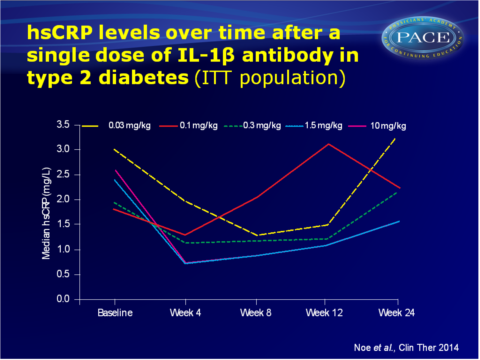
- A canakinumab dose-related reduction in hsCRP was seen at week 4 (-41%, -45%, -70% and -74% with 0.1, 0.3, 1.5 and 10 mg/kg canakinumab respectively, all P<0.05), as compared with placebo. Corresponding median changes in hsCRP values from baseline to week 4 were -0.2, -0.5, -1.5 and -1.7 mg/L.
- With the two highest doses, the reduction of hsCRP was maintained up to week 12 (-49% with 1.5 mg/kg and -60% with 10 mg/kg, both P<0.05, median changes from baseline: -0.8 and -1.3 mg/L respectively).
- After a single dose administration of 10 mg/kg canakinumab, 21 out of 27 patients (77.8) with baseline hsCRP>2 mg/L achieved hsCRP<2 mg/L, as compared with 3 out of 21 patients (14.3%) on placebo at week 4.
- At week 12, single dose administration of canakinumab 10 mg/kg was associated with a placebo-adjusted decrease in HbA1c of 0.31% (P=0.038).
- The incidence of reported adverse events was similar in canakinumab and placebo-treated patients, and no apparent trend was noted across the canakinumab dose range.
Download Noe clin Therap 2014 PACE.pptx
Conclusion
These data show that canakinumab use was associated with a dose-dependent reduction in hsCRP levels in patients with T2DM at week 4, which was maintained until week 12 at a dose of 1.5 or 10 mg/kg. These doses also showed a statistically significant, but clinically irrelevant reduction in HbA1c. A single injection of canakinumab, administered in a range of doses, was well tolerated. The sustained dampening of inflammatory response seen in this study suggests that quarterly dosing is feasible. The CANTOS outcomes trial is currently testing the hypothesis that long-term inhibition of IL-1β with canakinumab reduces the rate of recurrent CV events due to atherothrombosis. The current findings support the feasibility of the CANTOS trial.Find this article on Pubmed
References
1. Nissen SE,et al.Statin therapy,LDL cholesterol,C- reactive protein,and coronary artery disease. N EnglJ Med. 2005;352:29–38.
2. Ridker PM,et al.C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28.
3. Morrow DA,et al.Clinical relevance of C-reactive protein during follow-up of patients with acute coronary syndromes in the Aggrastat-to-ZocorTrial. Circulation. 2006;114:281– 288.
4. McMurray JJ,et al.Effects of statin therapy according to plasma high-sensitivity C-reactive protein concentration in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA): A retrospective analysis. Circulation. 2009;120:2188–2196.
5. Ridker PM,et al.Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182.
6. Church LD,McDermottMF.Canakinumab, a fully- human mAb against IL-1beta for the potential treatment of inflammatory disorders. Curr OpinMolTher. 2009;11: 81–89.
7. Dinarello CA,Donath MY,Mandrup-Poulsen T.Role of IL-1beta in type 2 diabetes. Curr OpinEndocrinolDiabetes Obes. 2010;17:314–321.
