LDL-hypothesis confirmed with non-statin therapy, even below target LDL-c levels
The IMPROVE-IT trial shows that lowering LDL-c with addition of the cholesterol absorption inhibitor ezetimibe to simvastatin further lowers CV events, in ACS patients with LDL-c levels within recommendations.
Ezetimibe Added to Statin Therapy after Acute Coronary SyndromesLiterature - Cannon et al., NEJM 2015
Cannon CP, Blazing MA, Giugliano RP, et al.
NEJM online June 3, 2015. DOI: 10.1056/NEJMoa1410489
Background
Intensive statin therapy incrementally reduces LDL-c levels as well as rates of nonfatal CV events, as compared with moderate dose statin therapy, but residual risk of recurrent CV events remains [1-5]. Moreover, due to safety concerns associated with high-dose statin therapy [6], additional lipid-modifying therapies are warranted.Ezetimibe targets the Niemann-Pick C1-like 1 (NPC1L1) protein, thereby inhibiting absorption of cholesterol from the intestine [7,8]. In addition to statins, ezetimibe lowers LDL-c with an extra mean 23-24% [9,10]. It was as yet unknown whether further lowering of LDL-c levels with addition of ezetimibe to statin therapy yields a clinical benefit.
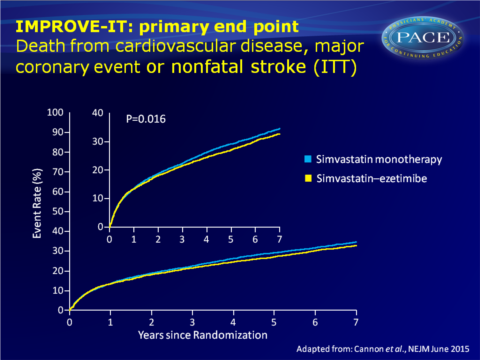
The Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) therefore evaluated the effect of ezetimibe combined with simvastatin (SIM-EZE), as compared with simvastatin monotherapy (SIM), in stable patients who had experienced an acute coronary syndrome (ACS), with LDL-c values within guideline recommendations. 18144 patients were randomised to SIM alone or SIM-EZE. After a median follow-up of 6 years, 42% of patients in both groups had discontinued the study medication without having experienced a primary end-point event or having died.
Main results
- Mean LDL-c at the time of hospitalisation for the index event was 93.8 mg/dL (2.4 mmol/L) in each group. Over the course of the trial, the median time-weighted average LDL-c was 69.5 mg/dL (1.8 mmol/L) in SIM-treated patients and 53.7 mg/dL (1.4 mmol/L) in patients on SIM-EZE (P<0.001).
- In those who had blood samples drawn after 1 year, SIM-EZE-treated patients showed on average 16.7 mg/dL (0.43 mmol/L) lower (P<0.001) LDL-c levels than SIM-treated patients.
- More SIM-EZE treated patients achieved the dual goal of LDL-c<70 mg/dL (1.8 mmol/L) and hsCRP < 2.0 at 1 month (50.6% vs. 30.5% with SIM alone).
- An absolute risk reduction of 2.0 percentage points for the primary end point at 7 years was seen with addition of ezetimibe (32.7% vs. 34.7%, HR: 0.936, 95%CI: 0.89-0.99, P=0.016), the benefit of which already appeared to emerge at 1 year.
- The rates of death from CV or any cause did not differ between the groups.
- Risk of any myocardial infarction (MI) was lower with addition of simvastatin (13.1% vs. 14.8%, HR: 0.87, 95%CI: 0.80-0.95, P=0.002), as was the risk of ischaemic stroke (3.4% VS. 4.1%, HR: 0.79, 95%CI: 0.67-0.94, P=0.008).
A non-significantly higher risk of haemorrhagic stroke was seen with addition of EZE (0.8% vs. 0.6%, HR: 0.93-2.04, P=0.11). - The benefit of addition of ezetimibe to simvastatin was consistent across almost all prespecified subgroups, and appeared particularly evident in patients with diabetes mellitus and in patients of 75 years or older.
- The frequency of pre-specified safety end points did not differ between treatment arms. 10.1% of patients treated with SIM monotherapy and 10.6% on SIM-EZE combination therapy discontinued treatment due to an adverse event.
Conclusion
Addition of the non-statin agent ezetimibe to simvastatin further lowered LDL-c by about 24% and yielded a statistically significantly lower risk of CV events, as compared with simvastatin monotherapy. No difference was seen in CV mortality or death by any cause between treatment groups, but rates of MI and ischaemic stroke were significantly lower with addition of ezetimibe.The clinical benefit obtained with ezetimibe is consistent with that seen with LDL-c lowering in statin trials. Although other lipoproteins and hsCRP were also reduced, the consistency with the observations in statin trials does support the LDL-hypothesis that predicts that lowering LDL-c leads to a reduction in CV events, even beyond guideline-recommended target of 70 mg/dL.
Editorial comment [11]
Despite a large body of evidence supporting the LDL hypothesis, in which LDL-c is a causal factor in the development of atherosclerotic vascular disease, some discussion remains on that the beneficial effects of statins are not adequately explained by their effects on LDL-c. An alternative theory, the ‘statin hypothesis’, proposes that the pleiotropic effects of statins that are not related to their lipid-lowering effect, may account for at least some of the benefit of statin therapy in preventing CV events; thus that statins have a unique efficacy in atherosclerotic vascular disease that is not shared by other lipid-modifying agents, and that lowering LDL-c levels is not the only basis for the beneficial effect of statins. “IMPROVE-IT is a landmark study in that it is the first clinical trial to show a benefit of adding a nonstatin lipid-modifying agent to statin therapy.” (…) “IMPROVE-IT should not be interpreted as showing anything uniquely beneficial about the use of ezetimibe. Indeed, the real implication of IMPROVE-IT is to suggest that all reductions in LDL levels, regardless of mechanism, are of equivalent benefit.” (…) “These data help emphasize the primacy of LDL cholesterol lowering as a strategy to prevent coronary heart disease. Perhaps the LDL hypothesis should now be considered the “LDL principle.”Find this article online at NEJM
References
1. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350: 1495- 504.
2. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004; 292: 1307-16.
3. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352: 1425-35.
4. Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294: 2437-45.
5. Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol 2006; 48: 438-45.
6. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensivedose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011; 305: 2556-64.
7. Sudhop T, Lutjohann D, Kodal A, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation 2002; 106: 1943-8.
8. Kosoglou T, Meyer I, Veltri EP, et al. Pharmacodynamic interaction between the new selective cholesterol absorption inhibitor ezetimibe and simvastatin. Br J Clin Pharmacol 2002; 54: 309-19.
9. Ballantyne CM, Blazing MA, King TR, Brady WE, Palmisano J. Efficacy and safety of ezetimibe co-administered with simvastatin compared with atorvastatin in adults with hypercholesterolemia. Am J Cardiol 2004; 93: 1487-94.
10. Morrone D, Weintraub WS, Toth PP, et al. Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: a pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis 2012; 223: 251-61.
11. Jarcho JA, Keany JF. Proof That Lower Is Better — LDL Cholesterol and IMPROVE-IT. NEJM 2015; June 3. DOI: 10.1056/NEJMe1507041
