Lomitapide reduces LDL-C in homozygous familial hypercholesterolaemia
In co-administration with other lipid-lowering therapies, the benefits of substantial and stable LDL-reduction by lomitapide may outweigh gastrointestinal side-effects + editorial comments
Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study.Literature - Cuchel M, Meagher EA, du Toit Theron H et al., Phase 3 HoFH Lomitapide Study investigators - Lancet. 2013 Jan 5;381(9860):40-6
Cuchel M, Meagher EA, du Toit Theron H et al., Phase 3 HoFH Lomitapide Study investigators
Lancet. 2013 Jan 5;381(9860):40-6. doi: 10.1016/S0140-6736(12)61731-0
Background
Patients suffering from homozygous familial hypercholesterolaemia, often caused by mutations affecting the function of the LDL-receptor (LDL-R), respond inadequately to conventional drug treatment, which are generally aimed at reducing LDL-C through upregulation of hepatic LDL-R. Current standard care therefore included LDL apheresis, which transiently reduces LDL-C by more than 50%. This can delay the onset of atherosclerosis [1,2]. However, LDL levels remain elevated despite drug therapy combined with apheresis, with persistently high cardiovascular risk [3].Lomitapide is an inhibitor of the microsomal triglyceride transport protein (MTP), a key protein in the assembly and secretion of ApoB-containing lipoproteins in the liver and intestine [4]. In a rabbit model for homozygous familial hypercholesterolaemia lomitapide substantially reduced LDL-C levels [5]. Oral intake for 18 weeks reduced LDL-C levels in 6 patients with homozygous familial hypercholesterolaemia, by reducing LDL-C production [6].
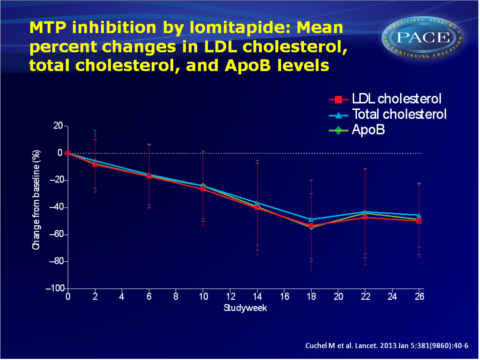
This study aimed to assess the long-term safety and efficacy of lomitapide when added to currently available lipid-lowering drug therapy with or without apheresis, during 78 weeks of treatment in adult patients with homozygous familial hypercholesterolaemia. This is a single-arm, phase 3, open-label, multi-centre study. Lomitapide was initiated at a low dose (5 mg) which was increased to an individualised maximum dose in the presence of a low-fat diet. In the efficacy phase mean percent change in LDL-C levels was determined in 29 patients at 26 weeks, after which 23 patients kept taking lomitapide until week 78 for safety assessment.
Main results
- Mean LDL-C significantly decreased by 50% (95%CI: -62 to -39) to 4.4 mmol/L from baseline (8.7 mmol/L) to the end of the efficacy phase (week 26). Secondary endpoints of total cholesterol, ApoB and triglyceride showed consistent results. At week 56, LDL-C levels were still reduced by 44% (95%CI: -57 to -31) and by 38% (95%CI: -52 to -24) by week 78.
- Based on the LDL-lowering response, 3 patients stopped LDL-apheresis altogether and 3 others increased the time interval between apheresis treatments. Despite this, lomitapide reduced LDL-C at week 78, as well as total cholesterol, ApoB, and triglyceride levels.
- HDL-C concentrations were significantly reduced at week 26, but had returned to levels similar to baseline by week 78.
- Most patients suffered from at least one adverse event during both the efficacy (27/29 patients) and the safety (21/23) phases, which were generally considered to be of mild to moderate intensity, and were mostly of gastrointestinal origin. 3 patients had discontinued the study by week 12 due to gastrointestinal disorders.
3 patients suffered from serious adverse events, which were considered unrelated or unlikely to be related to study treatment.
No serious adverse events were reported between week 26 and 78. - 10 patients showed elevated levels of ALT, AST or both at least once during the study, but this was never a reason to discontinue study treatment. Elevated liver-functions were managed by either dose reduction or temporary interruption of lomitapide according to protocol.
- Hepatic fat increased from 1.0% (range 0-5.0) at baseline to 8.6% (0-33.6) at week 26, 5.8% (0-16.5) at week 56 and 8.3% (0-19.0) at week 78.
Download PACE Cuchel lancet 2013.pptx
Conclusion
These findings show that lomitapide, when given concomitantly with other lipid-lowering therapies, significantly reduces LDL-C in patients with homozygous familial hypercholesterolaemia. Due to the rarity of the disease, studies of cardiovascular outcome are not feasible, but circumstantial evidence suggests that even a modest reduction in LDL-C levels reduces cardiovascular risk and improves survival.This was the first long-term study of any MTP inhibitor in men, which shows that lomitapide, in combination with a low-fat diet, was generally well-tolerated and stably and substantially reduced LDL-C levels. Despite some adverse events, the benefit-risk ratio of lomitapide seems favourable for patients with homozygous familial hypercholesterolaemia.
Editorial comment [7]
Inhibition of MTP is potentially a powerful therapeutic method to reduce ApoB-containing lipoproteins, and LDL-C was indeed reduced by 50% at a median dose of 40 mg lomitapide. As expected based on the mechanism of action, gastrointestinal side-effects were common. Despite limitations of the study set-up, the LDL-C reductions is impressive. Given the adverse effects and that lomitapide needs to be titrated to a maximally tolerated dose in individual patients, use of this drug will probably be limited to patients who are monitored in specialised lipid clinics.Since treatment will most likely be life-long, longer-term LDL-C reduction must be confirmed, and more importantly, progression of accumulation of liver fat to hepatic fibrosis or cirrhosis must be excluded.
Correspondence [8]
It is currently unknown whether the liver findings upon lomitapide treatment are associated with an increased risk of insulin resistance, systematic inflammation, liver fibrosis and cirrhoses, and hepatic cancer. Since there is some evidence that ezetimibe reduces liver fat, dr. Santos asks the researchers in a letter to the Lancet whether the authors have data on differences in the evolution of liver fat content between patients taking ezetimibe and those who do not. Also, have markers of insulin resistance been monitored over time? Also information from liver biopsies in those who accumulated the highest amounts of liver fat, can help determine if the newer drugs can indeed, in the long run, help patients with homozygous familial hypercholesterolaemia.Correspondence: response from the authors [9]
The authors agree that long-term consequences of liver fat accumulation must be closely monitored in clinical practice. Insulin concentrations were not measured, but fasting plasma glucose were unaffected. In one patient, two liver biopsies were performed, of which the one taken after 2 years of treatment with 40 mg lomitapide showed a modest increase in steatosis as compared to baseline, without inflammation or fibrosis.Concerning co-administration of ezetimibe, they refer to a study by Samaha, in which treatment with lomitapide 10 mg and ezetimibe 10 mg more efficiently reduced LDL-C than did lomitapide monotherapy, but had the same frequency of aminotransferase elevation. Hepatic fat was not assessed.
It is unclear whether hepatic fat is causally related to insulin resistance, therefore the consequences of lomitapide-induced hepatic fat remain unknown. The recent approval of lomitapide by the FDA calls for a systematic addressing of these long-term questions about hepatic safety.
References
1. Hudgins LC, Kleinman B, Scheuer A, et al. Long-term safety and effi cacy of low-density lipoprotein apheresis
in childhood for homozygous familial hypercholesterolemia. Am J Cardiol 2008; 102: 1199–204.
2. Thompson GR, Barbir M, Davies D, et al. Efficacy criteria and cholesterol targets for LDL apheresis. Atherosclerosis 2010; 208: 317–21.
3. Thompson GR, Catapano A, Saheb S, et al. Severe hypercholesterolaemia: therapeutic goals and eligibility criteria for LDL apheresis in Europe. Curr Opin Lipidol 2010; 21: 492–98.
4. Wetterau JR, Lin MC, Jamil H. Microsomal triglyceride transfer protein. Biochim Biophys Acta 1997; 1345: 136–50.
5. Wetterau JR, Gregg RE, Harrity TW, et al. An MTP inhibitor that normalizes atherogenic lipoprotein levels in WHHL rabbits. Science 1998; 282: 751–54.
6. Cuchel M, Bloedon LT, Szapary PO, et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med 2007; 356: 148–56.
7. Raal FJ. Lomitapide for homozygous familial hypercholesterolaemia. Lancet. 2013 Jan 5;381(9860):7-8.
8. Santos RD. Lipid-lowering treatment for homozygous familial hypercholesterolaemia. Lancet. 2013 Apr 6; 381 (9873):1182
9. Cuchel M. Lipid-lowering treatment for homozygous familial hypercholesterolaemia – Authors' reply. Lancet. 2013 Apr 6; 381 (9873):1183
