Meta-analysis of large NOAC trials shows favourable risk/benefit ratio over warfarin
Combined data from RE-LY, ROCKET-AF, ARISTOTLE and ENGAGE AF-TIMI 48 showed that NOACs safely reduce stroke and systemic embolism in comparison with warfarin, in atrial fibrillation.
Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trialsLiterature - Ruff et al., The Lancet 2013 - The Lancet, 4 December 2013
Ruff CT, Giugliano RP, Braunwald E, et al.
The Lancet, Early Online Publication, 4 December 2013 doi:10.1016/S0140-6736(13)62343-0
Background
Atrial fibrillation (AF) comes with an increased risk of embolic stroke and has a higher mortality than sinus rhythm [1,2]. Warfarin and other vitamin K antagonists (VKAs) are effective in the prevention of thromboembolism, but their use requires frequent monitoring and dose adjustments due to the narrow therapeutic index. The associated risk and inconvenience translates into poor adherence, which may explain the systematic underuse of VKAs for stroke prevention [3,4].Several new oral anticoagulants (NOACs) now exist that offer potential advantages over VKAs, including rapid onset and offset of the effect, absence of an effect of dietary vitamin K on their activity, and fewer drug interactions. The more predictable anticoagulant effects of the new drugs allow the use of fixed doses without the need for routine monitoring of coagulation.
Individually, the NOACs have been found to be at least as safe and effective as warfarin in the prevention of stroke and systemic embolism in patients with AF [5-8]. Dabigatran, rivaroxaban and apixaban have been approved by regulatory authorities, while edoxaban has just completed late-stage clinical assessment.
This is the first meta-analysis comparing NOACs with warfarin that includes data from the Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction study 48 (ENGAGE AF-TIMI 48) [8,9], which tested edoxaban. Data from the RE-LY (dabigatran)[5], ROCKET-AF (rivaroxaban)[6] and ARISTOTLE (apixaban)[7] were combined, amounting to 42411 participants who received a NOAC and 29272 who received warfarin. This analysis aims to better define the balance between efficacy and safety in important clinical subgroups, and the effects of these agents on important secondary outcomes.
Main results
- Allocation to a NOAC significantly reduced the composite of stroke or systemic embolic events by 19% as compared to warfarin (RR on combined data: 0.81, 95%CI: 0.73-0.91), P<0.0001).
- The overall beneficial effect was mainly driven by a large reduction in haemorrhagic stroke (RR on combined data: 0.49, 95%CI: 0.38-0.64, P<0.0001). All-cause mortality was also significantly reduced with NOACs vs. warfarin (RR: 0.90, 95%CI: 0.85-0.95, P=0.0003), while ischemic stroke and myocardial infarction were not.
- High-dose NOACs gave a non-significant reduction in major bleeding (RR:0.86, 95%CI: 0.73-1.00, P=0.06). A substantial reduction in intracranial haemorrhage was observed (RR: 0.48, 95%CI: 0.39-0.59, P<0.0001). NOACs were associated with a higher rate of gastrointestinal bleedings (RR: 1.25, 95%CI: 1.01-1.55, P=0.0430).
- The benefit of NOACs in comparison with warfarin in reduction stroke or systemic embolism was consistent across all subgroups examined (based on age, sex, with or without diabetes, previous stroke or TIA, creatinine clearance, VKA status, centre-based TTR and different CHADS2 scores).
- Safety of NOACs compared with warfarin was generally consistent across subgroups for the reduction of major bleedings, with the exception of a significant interaction for centre-based time in therapeutic range (TTR), with a larger relative reduction in bleedings seen in centres that achieved a TTR of less than 66% as compared to >66%.
- Low-dose NOAC regimes had similar efficacy to warfarin for the composite of stroke or systemic embolic events. Low-dose regimens were associated with a non-significant reduction in major bleeding, but with significantly fewer intracranial haemorrhages. Gastro-intestinal bleeding rate was similar between low-dose NOACs and warfarin.
- Analysis of only the factor Xa inhibitors (thus exclusion of dabigatran) gave similar results to the main analysis, for both stroke or systemic embolic events and major bleedings.
Download Ruff 2013 pace.pptx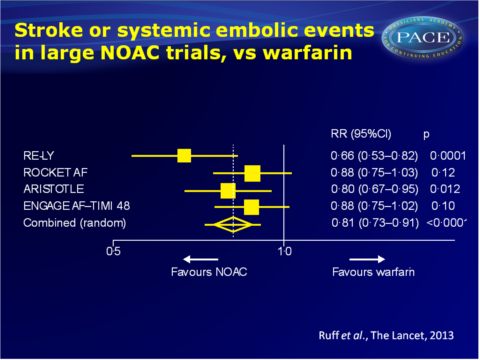


Conclusion
Patients receiving high-dose NOACs had fewer stroke and systemic embolic events than patients on warfarin, a result mainly driven by substantial protection against haemorrhagic stroke. The NOACs had an overall favourable safety profile as compared with warfarin, although they are associated with an increase in gastro-intestinal bleeding. A reduced all-cause mortality is observed with NOACs in comparison with warfarin.The two low-dose NOAC regimes were as effective for protection against all stroke or systemic embolic events, but not as effective for protection against ischemic stroke. They have a safer profile than warfarin and show a similar mortality benefit to the high-dose regimens.
The data were pooled at the study level, since individual participant data were not available. Factor Xa inhibitors (rivaroxaban, apixaban, edoxaban) and the thrombin inhibitor dabigatran were pooled. The authors consider this justified because all drugs are specific inhibitors of factors in the coagulation cascade, and because the trials were qualitatively similar in design. Analysis of only factor Xa inhibitors indeed gave similar results to the overall analysis. Some statistical heterogeneity across the trials with respect to major bleeding and gastro-intestinal bleeding, which might reflect differences between drugs.
The favourable balance for NOACs between efficacy and safety compared with warfarin was consistent across a wide range of patients with atrial fibrillation known to be at high risk for both ischemic and bleeding events.
Editorial comment [10]
“Such a meta-analysis assumes that all the novel oral anticoagulant drugs are the same (which theyare not) and work on the basis of a class effect or are broadly equivalent; and that the randomised trials are homogeneous, which again they are not. Ruff and colleagues’ meta-analysis does not really answer the question of which novel oral anticoagulant is best, whether from an efficacy or safety perspective. Indirect comparisons between novel oral anticoagulants suggest that, ultimately, the drug could be fitted to the patient, or the patient to the drug, dependent on a focus on safety or efficacy, and on other patient factors, such as renal function and drug compliance. A major dilemma of clinical management is how to predict newly diagnosed non-anticoagulated patients with atrial fibrillation who would do well on vitamin K antagonists with high time in therapeutic range, becausethe main benefits of novel oral anticoagulants compared with warfarin might be only marginal in those with high times in therapeutic range, although the reduction in intracranial haemorrhage is still evident.”References
1 Camm AJ, Kirchhof P, Lip GY, et al, and the European Heart Rhythm Association, and the European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fi brillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31: 2369–429.
2 Hylek EM, Go AS, Chang Y, et al. Eff ect of intensity of oral anticoagulation on stroke severity and mortality in atrial fi brillation. N Engl J Med 2003; 349: 1019–26.
3 Birman-Deych E, Radford MJ, Nilasena DS, Gage BF. Use and effectiveness of warfarin in Medicare benefi ciaries with atrial fi brillation. Stroke 2006; 37: 1070–74.
4 Hylek EM, Evans-Molina C, Shea C, et al. Major hemorrhage and tolerability of warfarin in the fi rst year of therapy among elderly patients with atrial fi brillation. Circulation 2007; 115: 2689–96.
5 Connolly SJ, Ezekowitz MD, Yusuf S, et al, and the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fi brillation. N Engl J Med 2009; 361: 1139–51.
6 Patel MR, Mahaff ey KW, Garg J, et al, and the ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–91.
7 Granger CB, Alexander JH, McMurray JJ, et al, and the ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fi brillation. N Engl J Med 2011; 365: 981–92.
8 Giugliano RP, Ruff CT, Braunwald E, et al. Once-daily edoxaban versus warfarin in patients with atrial fi brillation. N Engl J Med 2013; 369: 2093–104.
9 Ruff CT, Giugliano RP, Antman EM, et al. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fi brillation: design and rationale for the Effective aNticoaGulation with factor xA next GEneration in Atrial Fibrillation-Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF-TIMI 48). Am Heart J 2010; 160: 635–41.
10 Larsen B, Lip GYH. Warfarin or novel oral anticoagulants for atrial fi brillation? The Lancet. December 4, 2013
http://dx.doi.org/10.1016/S0140-6736(13)62376-4
