Mipomersen as add-on therapy in FH
18/10/2012
Circulation, Oct 2012 . Does mipomersen fill an unmet medical need in Heterozygous familial hypercholesterolemia?
Apolipoprotein B Synthesis Inhibition with Mipomersen in Heterozygous Familial Hypercholesterolemia: Results of a Randomized, Double-Blind, Placebo Controlled Trial to Assess Efficacy and Safety as Add-on Therapy in Patients with Coronary Artery Disease.Literature - Stein EA, Dufour R, Gagne C, et al. Circulation. 2012 Oct 11
Stein EA, Dufour R, Gagne C, et al.
Circulation. 2012 Oct 11. [Epub]
Heterozygous familial hypercholesterolemia (HeFH) is a common genetic disorder with a
prevalence of 1 in 500 and associated with significant cardiovascular mortality and morbidity [1].
The most common cause of HeFH are loss-of-function mutations in LDL-receptor alleles [2] leading to markedly elevated LDL cholesterol and early cardiovascular disease with early onset (<50 years in men, < 60 years in women)[3]. Despite of effective therapies, a need remains for additional LDL-lowering therapies. Mipomersen inhibits apolipoprotein B-100 protein synthesis in the liver and release of apoB-containing lipoproteins into the circulation [4].
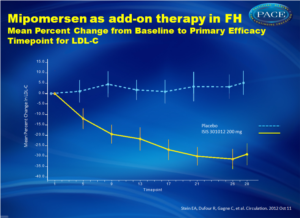
This study assessed the efficacy and safety of mipomersen added to maximally tolerated lipid lowering therapies in patients with HeFH and CAD. Weekly mipomersen 200 mg s.c. was compared with placebo for 26 weeks. The primary endpoint was percent change in LDL-C from baseline at week 28.
While clinical benefits of these additional reductions in apolipoprotein B and associated atherogenic lipids remain to be proven, mipomersen may fill an unmet medical need in HeFH.
Animation: Lp(a) is an atherogenic, ApoB-containing lipoprotein that consists of an LDL particle in which the ApoB molecule is attached to a separate variably sized apolipoprotein called apo(a)....
Read more...
Circulation. 2012 Oct 11. [Epub]
Background
Heterozygous familial hypercholesterolemia (HeFH) is a common genetic disorder with aprevalence of 1 in 500 and associated with significant cardiovascular mortality and morbidity [1].
The most common cause of HeFH are loss-of-function mutations in LDL-receptor alleles [2] leading to markedly elevated LDL cholesterol and early cardiovascular disease with early onset (<50 years in men, < 60 years in women)[3]. Despite of effective therapies, a need remains for additional LDL-lowering therapies. Mipomersen inhibits apolipoprotein B-100 protein synthesis in the liver and release of apoB-containing lipoproteins into the circulation [4].
This study assessed the efficacy and safety of mipomersen added to maximally tolerated lipid lowering therapies in patients with HeFH and CAD. Weekly mipomersen 200 mg s.c. was compared with placebo for 26 weeks. The primary endpoint was percent change in LDL-C from baseline at week 28.
Main results
- Mipomersen treatment resulted in a statistically significant mean percent reduction from baseline in LDL cholesterol (-28.0%) compared to a small increase with placebo (+5.2%) (P<0.001).
- The reduction in LDL cholesterol was independent of baseline LDL cholesterol level and age but the response in females was greater than in males (mean +SD, -40.6% [20.5%] v.-20.0% [27.7%], respectively) although the effect in males was still statistically significant. No gender difference was observed in the placebo group.
- The mean percent change in apolipoprotein B was significantly greater with mipomersen treatment (-26.3%) compared to placebo (+7.0%), P<0.001 and was also greater in females than males in the mipomersen group.
- Adverse events included injection site reactions and flu-like symptoms. Five (6%) mipomersen patients had 2 consecutive alanine aminotransferases ≥3 times ULN at least 7 days apart; none were associated with significant bilirubin increases. Hepatic fat content increased a median of 4.9% with mipomersen vs. 0.4% with placebo, P<0.001.
Conclusion
Mipomersen provided significant additional reductions in apolipoprotein B associated atherogenic lipoproteins in HeFH patients with CAD, already maximally treated with current lipid-lowering drugs. Adverse effects were mainly injection site reactions, flu-like symptoms, and increased hepatic fat and ALT/AST, the clinical significance of which remain uncertain.While clinical benefits of these additional reductions in apolipoprotein B and associated atherogenic lipids remain to be proven, mipomersen may fill an unmet medical need in HeFH.

Lipoprotein (a)
Animation: Lp(a) is an atherogenic, ApoB-containing lipoprotein that consists of an LDL particle in which the ApoB molecule is attached to a separate variably sized apolipoprotein called apo(a)....
Read more...