Monotherapy with PCSK9-antibody superior to ezetimibe in hypercholesterolaemia
Phase III study shows that monotherapy with alirocumab, a PCSK9 antibody, is more effective at lowering LDL-c than ezetimibe, in patients with hypercholesterolaemia and moderate CV risk.
Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: Results of a 24 week, double-blind, randomized Phase 3 trialLiterature - Roth EM et al., Int J Cardiol. 2014 - Int J Cardiol. 2014 Jul 2
Roth EM, Taskinen MR, Ginsberg HN et al.
Int J Cardiol. 2014 Jul 2. doi: 10.1016/j.ijcard.2014.06.049. [Epub ahead of print]
Background
Alirocumab is a fully human monoclonal antibody against proprotein convertase subtilisin/kexin 9 (PCSK9), which was shown to reduce LDL-c in combination with other lipid-lowering therapies in phase II studies of 8-12 weeks duration [1-3]. All patients in these studies also received background statin therapy.Statins increase PCSK9 levels, thus it is important to study alirocumab as monotherapy, to better understand the pharmacokinetics and pharmacodynamics of the drug, and the efficacy and safety in patients not receiving statin therapy.
The ODYSSEY MONO study evaluated the efficacy and safety of alirocumab monotherapy, in comparison with ezetimibe, in patients with hypercholesterolaemia at moderate cardiovascular (CV) risk (10-year risk of fatal CV events >1% and <5%) [4]. Patients were not on statin or other lipid-lowering therapy. Alirocumab was given in a dose regimen of 75 mg every 2 weeks (Q2W, self-injection). This study is part of the ODYSSEY program, a large series of phase III studies designed to comprehensively assess the efficacy and safety of alirocumab in a range of clinical settings and patient groups.
103 patients were randomised to alirocumab or ezetimibe. 14 patients in the alirocumab arm were up-titrated at week 12 to 150 mg Q2W in a blinded manner, since their LDL-c was >70 mg/dL in week 8. 44/52 (85%) patients in the alirocumab arm and 44/51 (86%) patients in the ezetimibe arm completed the 24-week treatment period.
Main results
- In the primary efficacy analysis (intention to treat), least-squares (LS) mean percent reductions in LDL-c from baseline to week 24 were 47 (+SE:3) % with alirocumab vs. 16 (+3) % in the ezetimibe group, meaning a statistically significant difference between groups of -32 (+4) % (P<0.0001). On-treatment analysis gave consistent results.
- In week 12, when all patients in the alirocumab arm received 75 mg Q2W, LDL-c levels were reduced by 48 (+3) %, vs. 20 (+3)% with ezetimibe in the ITT analysis, with an LS mean difference of -28 (+4) % between groups (P<0.0001). On-treatment analysis showed similar effects.
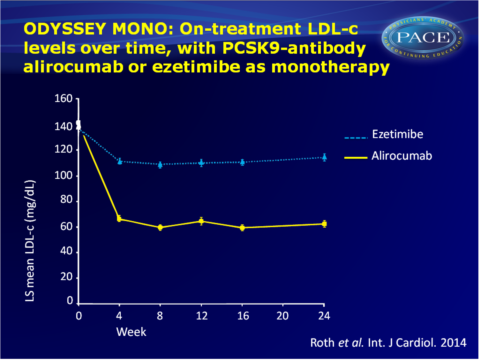
- The time-course of changes in LDL-c levels showed a substantial drop from baseline to week 4 in patients receiving alirocumab, after which reduction remained stable until the end of the study. Ezetimibe showed a smaller drop at week 4, which was maintained until week 24.
- Percent reductions from baseline in apolipoprotein B, total cholesterol and non-HDL-c were significantly greater in patients on alirocumab than on ezetimibe at week 24. Moderate reductions in lipoprotein (a), triglycerides and increases in HDL-c were seen after both treatments, without differing significantly.
- Overall, 69% of patients in the alirocumab group and 78% in the ezetimibe group experienced at least one treatment-emergent adverse event (TEAEs). One serious adverse event was seen in each group, and 9 patients (5 on alirocumab, 4 on ezetimibe) discontinued study treatment due to TEAEs. Other adverse events were also generally similar in the two treatment groups.
Six (12%) of patients on alirocumab showed anti-drug antibodies. Titres were low and no neutralising anti-drug antibody that may impact alirocumab pharmacokinetics, LDL-c effects or safety was detected.
Download Roth int card 2014 PACE.pptx
Conclusion
This phase III study of alirocumab showed that it had superior efficacy as monotherapy, as compared with ezetimibe, over 24 weeks of treatment. The findings suggests that 75 mg Q2W is sufficient to provide >50% LDL-c reduction in most patients with moderate CV risk. Tolerability and safety of alirocumab was comparable with ezetimibe, which is relevant for statin intolerant people.Find this article on Pubmed
References
1. McKenney JM, Koren MJ, Kereiakes DJ, et al. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol 2012;59:2344–53.
2. Roth EM, McKenney JM, Hanotin C, et al. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia.NEngl JMed 2012;367:1891–900.
3. Stein EA, Gipe D, Bergeron J, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet 2012;380:29–36.
4. Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987–1003.
