New CETP-inhibitor potently lowers atherogenic lipoproteins in phase 2 trial
The well tolerated CETP inhibitor TA-8995 substantially reduced LDL-c and apoB, while raising HDL-c and apoA1 and promoting cholesterol efflux in the TULIP trial.
Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP): a randomised, double-blind, placebo-controlled phase 2 trialLiterature - Hovingh GK et al., Lancet. 2015
Hovingh GK, Kastelein JJ, van Deventer SJ
Lancet. 2015 Jun 2. doi: 10.1016/S0140-6736(15)60158-1
Background
Despite statin and other lipid-lowering treatments, many people still have high LDL-c levels, and remain at high residual risk of cardiovascular events. Thus, efforts are directed at the development of novel drugs that provide additional lowering of LDL-c, when co-administered with a statin.One of the LDL-lowering strategies being explored is inhibition of cholesteryl ester transfer protein (CETP), since it transfers cholesterol into atherogenic LDL particles [1]. Although initial human clinical studies did not show a cardioprotective effect of CETP inhibition with torcetrapib and dalcetrapib, the hypothesised beneficial effect of CETP lowering is currently being studied in two large clinical outcome trials, namely REVEAL and ACCELERATE. These trials evaluate CETP inhibitors that do not have the adverse off-target effects seen with torcetrapib.
TA-8995 is a novel CETP inhibitor that exerted potent effects on both pro-atherogenic and anti-atherogenic lipoprotein particles in a phase 1 study [2], without the off-target effects of torcetrapib. This randomised double-blind phase 2 12-week study assessed the safety and efficacy of TA-8995 as monotherapy or in combination with statins in 364 patients with mild dyslipidaemia (fasting LDL-c: 2.5-4.5 mmol/L, HDL-c: 0.8-1.8 mmol/L and triglyceride<4.5 mmol/L after run-in or wash-out of previous lipid-lowering treatments). Different doses of TA-8995 and combinations with statins were evaluated.
Main results
- After 12 weeks of treatment with TA-8995, LDL-c levels were significantly reduced by 27.4% in those receiving 1 mg, 32.7% in those on 2.5 mg, 45.3% in those on 5 mg and 45.3% in those given 10 mg.
Reductions on 5 and 10 mg were similar to reductions seen with atorvastatin 20 mg or rosuvastatin 10 mg, and addition of 10 mg TA-8995 to a statin gave up to 68.2% lower LDL-c. - 95% of patients receiving TA-8995 (5 or 10 mg daily) achieved LDL-c < 2.5 mmol/L, and 65% of patients on 5 mg and 63% on 10 mg TA-8995 reached LDL-c<1.8 mmol/L.
- At 12 weeks, HDL-c had increased significantly by 75.8% in those on 1 mg TA-8995, 124.3% in those on 2.5 mg, 157.1% in those on 5 mg, and 179.0% in those given 10 mg.
TA-8995 was equally effective as monotherapy or when given together with statins. - TA-8995 did not significantly affect plasma triglyceride levels or total cholesterol, but dose-dependent increases in apoA-1 and apoE concentrations were observed, apoB was significantly lower as compared with baseline.
- An exploratory analysis of cholesterol efflux capacity showed a dose-dependent increase in cholesterol efflux with serum from patients treated with TA-8995, while it was mildly reduced after 12 weeks in patients who received placebo.
- TA-8995 was well tolerated and the percentage of patients discontinuing due to adverse events was low and similar between treatment arms. Nasopharyngitis and headache were most commonly reported with TA-8995 monotherapy.
No effects of TA-8995 on laboratory safety parameters were noted. - TA-8995 concentrations decreased rapidly after discontinuation, and 8 weeks after the last dose, the concentration was less than 3% of the trough levels during treatment.
Download Hovingh Lancet 2015 PACE.pptx
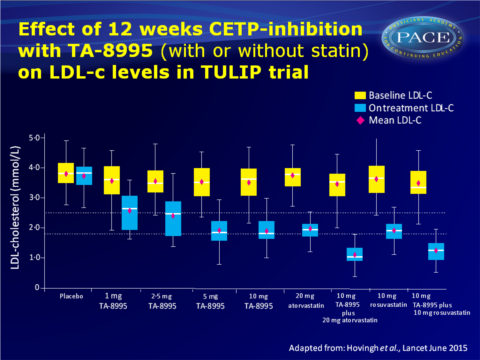
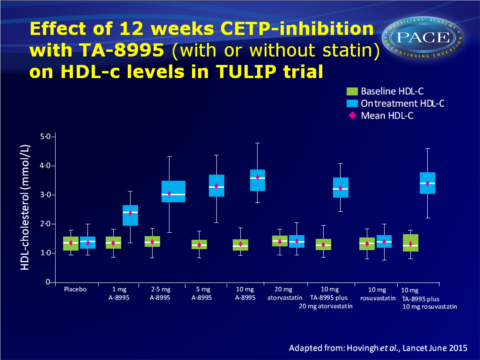
Conclusion
Treatment with the new, potent CETP-inhibitor TA-8995 lowered LDL-c (~45%) and apoB (~33%) levels, while increasing HDL-c (up to ~179%) and apoA-1 (up to ~63%) concentrations. When combined with statins, TA-8995 yielded an additional decrease of LDL-c of up to 50%.Although CETP-inhibitors were initially developed to raise HDL-c levels, TA-8995 potently lowers levels of atherogenic lipoproteins. The increase of plasma apoA-I concentrations and the ability to promote cholesterol efflux may represent additional benefits. The translation of the antiatherogenic effects of TA-8995 into a reduction of future CV events has to be tested in an outcome trial.
Editorial comment [3]
Previous studies with CETP inhibitors torcetrapib and dalcetrapib suggested “that CETP inhibition can be safe but that, in the absence of LDL cholesterol reduction, increasing cholesterol efflux by about 9% will not translate into reductions in cardiovascular disease.” Currently, evacetrapib and anacetrapib are being evaluated in two CV outcome studies. “Unlike dalcetrapib, both compounds increase ABCA1-mediated efflux from generation of lipid-poor prebeta HDL, and decrease lipoprotein(a). “Provided that current trials are sufficiently powered and no harmful effects emerge, then these treatments, by virtue of their LDL cholesterol-lowering potential alone, should reduce cardiovascular disease at least as favourably as ezetimibe in the IMPROVE-IT trial.”The TULIP investigators report that “TA-8995 inhibited CETP activity in a dose-dependent manner, and that this results in both a decrease in LDL cholesterol and an increase in HDL cholesterol, both as monotherapy and against a background of moderate intensity statin treatment.” (…) “The magnitude of the reduction in LDL cholesterol and lipoprotein(a) achieved by TA-8995 means that this oral compound is comparable with the potent subcutaneous PCSK9 inhibitors.”
Find this article online at The Lancet
References
1.Barter PJ, Rye KA. Cholesteryl ester transfer protein inhibition as a strategy to reduce cardiovascular risk. J Lipid Res 2012; 53:1755–66.
2 Ford J, Lawson M, Fowler D, et al. Tolerability, pharmacokinetics and pharmacodynamics of TA-8995, a selective cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjects. Br J Clin Pharmacol 2014; 78: 498–508.
3. Ray KK, Vallejo-Vaz AJ. The evolving role of CETP inhibition: beyond HDL cholesterol. The Lancet. June 3, 2015 http://dx.doi.org/10.1016/S0140-6736(15)60608-0
