New data on dabigatran for prevention of VTE
22/02/2013
NEJM Feb 2013. Results on extended prophylaxis with dabigatran in patients who have had venous thromboembolism (VTE).
Extended Use of Dabigatran, Warfarin or Placebo in Venous ThromboembolismLiterature -
Two studies published in the New England Journal of Medicine on extended prophylaxis with dabigatran in patients who have had venous thromboembolism (VTE).
Schulman S, Kearon O, Kakkar AK, et al.N Engl J Med. 2013;368:709-18.
Background
Anticoagulant treatment with vitamin K antagonists is recommended for patients with venous thromboembolism. Most patients receive at least 3 months of treatment; long-term treatment is recommended if there are risk factors for recurrence, such as multiple thrombotic episodes [1]. The risk of major bleeding is approximately 1% per year with extended vitamin K antagonist therapy after venous thromboembolism [2]. The risk of major bleeding, together with the need for frequent laboratory monitoring and dose adjustments, makes long-term treatment problematic.Dabigatran is a direct thrombin inhibitor which does not require frequent monitoring and dose adjustments. For the initial 6-month treatment of venous thromboembolism it was shown to be noninferior to warfarin and was associated with a lower rate of clinically relevant nonmajor bleeding [3]. Dabigatran might therefore also be suitable for extended therapy beyond the initial period of treatment.
In the RE-MEDY and the RE-SONATE trials, the role of dabigatran was examined in patients who had completed at least 3 months of initial VTE therapy. In RE-MEDY, the active-control study, 2,866 patients felt to be at increased risk for recurrent VTE were randomized to warfarin or dabigatran. In RE-SONATE, the placebo-control study, 1,353 patients were randomized to either dabigatran or placebo.
Main results:
In both studies, dabigatran was effective at preventing recurrent VTE.Recurrent VTE (fig. 1):
- 1.8% for dabigatran versus 1.3% for warfarin, HR 1.44 (0.78-2.64)
- 0.4% for dabigatran versus 5.6% for placebo, HR 0.08 (0.02-0.25)
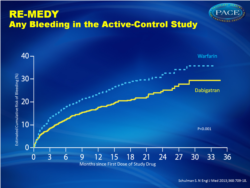
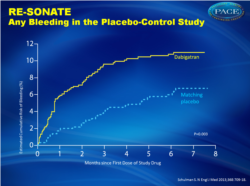
Major or clinically relevant bleeding (fig. 2):
- 5.6% for dabigatran versus 10.2% for warfarin, HR 0.54 (0.41-0.71)
- 5.3% for dabigatran versus 1.8% for placebo, HR 2.92 (1.52-5.60)
Conclusion:
Dabigatran 150 mg twice daily is an effective treatment option with a favorable safety profile for the extended prevention of recurrent venous thromboembolism after a first VTE.
Editorial comment [4]:
“For patients at higher risk, the new oral anticoagulants are attractive alternatives to warfarin; bleeding risk however must be considered. Improved risk stratification strategies are needed to identify patients who have the greatest risk of recurrence. These are the patients who stand to benefit most from extended anticoagulant treatment of a first unprovoked episode of venous thromboembolism.”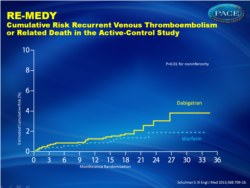 Figure 1-aCumulative Risk Recurrent Venous Thromboembolism or Related Death in the Active-Control Study |
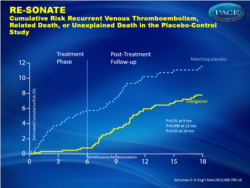 Figure 1 bCumulative Risk Recurrent Venous Thromboembolism, Related Death, or Unexplained Death in the Placebo-Control Study |
References:
1. Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease. Chest 2008;133:Suppl:454S-545S. [Erratum, Chest 2008;134:892.]2. Ost D, Tepper J, Mihara H, et al. Duration of anticoagulation following venous thromboembolism: a meta-analysis. JAMA 2005;294:706-15.
3. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342-52.
4. Connors JM. Extended Treatment of Venous Thromboembolism N Engl J Med 2013; 368:767-769
Abstract
Background:Dabigatran, which is administered in a fixed dose and does not require laboratory monitoring, may be suitable for extended treatment of venous thromboembolism.
Methods:
In two double-blind, randomized trials, we compared dabigatran at a dose of 150 mg
twice daily with warfarin (active-control study) or with placebo (placebo-control study) in patients with venous thromboembolism who had completed at least 3 initial months of therapy.
Results:
In the active-control study, recurrent venous thromboembolism occurred in 26 of 1430 patients in the dabigatran group (1.8%) and 18 of 1426 patients in the warfarin group (1.3%) (hazard ratio with dabigatran, 1.44; 95% confidence interval [CI], 0.78 to 2.64; P = 0.01 for noninferiority). Major bleeding occurred in 13 patients in the dabigatran group (0.9%) and 25 patients in the warfarin group (1.8%) (hazard ratio, 0.52; 95% CI, 0.27 to 1.02). Major or clinically relevant bleeding was less frequent with dabigatran (hazard ratio, 0.54; 95% CI, 0.41 to 0.71). Acute coronary syndromes occurred in 13 patients in the dabigatran group (0.9%) and 3 patients in the warfarin group (0.2%) (P = 0.02). In the placebo-control study, recurrent venous thromboembolism occurred in 3 of 681 patients in the dabigatran group (0.4%) and 37 of 662 patients in the placebo group (5.6%) (hazard ratio, 0.08; 95% CI, 0.02 to 0.25; P<0.001). Major bleeding occurred in 2 patients in the dabigatran group (0.3%) and 0 patients in the placebo group. Major or clinically relevant bleeding occurred in 36 patients in the dabigatran group (5.3%) and 12 patients in the placebo group (1.8%) (hazard ratio, 2.92; 95% CI, 1.52 to 5.60). Acute coronary syndromes occurred in 1 patient each in the dabigatran and placebo groups.
Conclusions:
Dabigatran was effective in the extended treatment of venous thromboembolism and carried a lower risk of major or clinically relevant bleeding than warfarin but a higher risk than placebo.