New diabetes stratification predicts risk of complications
In a data-driven cluster analysis of 6 variables in adult patients with newly diagnosed diabetes, 5 categories of patients were identified with different characteristics and risks of complications.
Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variablesLiterature - Ahlqvist E, Storm P, Käräjämäki A, et al. - Lancet Diab Endocrinol 2018; published online ahead of print
Background
Diabetes is diagnosed based solely on glucose levels, and classified simply into type 1 and type 2. Current guidelines do not provide criteria for intensified treatment of patients at high risk for complications. However, diabetes has a heterogeneous clinical presentation and progression, and several rare monogenic forms have been described [2-4].
In this study, a data-driven cluster analysis of six commonly measured variables was performed in 14,755 adult patients with newly diagnosed diabetes, in order to optimize their classification and identify those at high risk for complications. Data of the Swedish All new Diabetics in Scania cohort (ANDIS) were used for the cluster analysis, and replication was performed in three independent cohorts (the Scania Diabetes Registry (SDR), All New Diabetics in Uppsala (ANDIU) and Diabetes Registry Vaasa (DIREVA)). The Malmo Diet and Cancer CardioVascular Arm (MDC-CVA) was used as a non-diabetic cohort to study genetic loci previously associated with diabetes and related traits, by comparing each of the clusters in the ANDIS cohort with the MDC-CVA cohort.
The following variables were related to prospective development of complications and prescription of medications:
- glutamate decarboxylase antibodies (GADA)
- age at diagnosis
- body-mass-index (BMI)
- HbA1c
- β-cell function
- insulin resistance
Main results
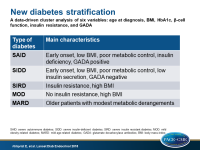
Based on the ANDIS cohort, 5 clusters were formed:
- Cluster 1 (N=577) was characterized by early-onset disease, relatively low BMI, poor metabolic control, insulin deficiency, and presence of GADA, and was labelled as severe autoimmune diabetes (SAID).
- Cluster 2 (N=1,575) was characterized by low age at onset, relatively low BMI, low insulin secretion (low HOMA2-B index), poor metabolic control, and was GADA negative, and was labelled as severe insulin-deficient diabetes (SIDD).
- Cluster 3 (N=1,373), was characterized by insulin resistance (high HOMA2-IR index) and high BMI, and was labelled as severe insulin resistant diabetes (SIRD).
- Cluster 4 (N=1,942), was characterized by obesity but not by insulin resistance, and was labelled as mild obesity-related diabetes (MOD).
- Cluster 5 (N=3,513), was characterized by older patients with modest metabolic derangements, and was labelled as mild age-related diabetes (MARD)
Clustering in the independent replication cohorts yielded similar results
In ANDIS, significant associations were observed between clusters and diabetic complications:
- Clusters 1 and 2 had higher HbA1c at diagnosis than other clusters, and this observation persisted throughout follow-up.
- Ketoacidosis at diagnosis was most frequent in cluster 1 (124/406, 31%) and cluster 2 (259/1033, 25% vs <5% in other clusters). HbA1c was the strongest predictor of ketoacidosis at diagnosis (OR: 2.73, 95%CI: 2.47-3.03, P<0.0001 per 1 SD change).
- Compared to cluster 5, time to sustained insulin use was shortest in cluster 1 (HR: 26.87; 95%CI: 21.17–34.11), followed by cluster 2 (HR: 10.97; 8.73–13.77).
- Patients in cluster 3 had the highest risk of developing chronic kidney disease in the ANDIS, SDR and DIREVA cohorts. In the ANDIS cohort, patients in cluster 2 were more likely to develop early signs of diabetic retinopathy (for example, OR vs cluster 5: 1.6; 95%CI: 1.3–1.9; P<0.0001), which was replicated in the ANDIU and SDR cohorts
- Analysis of genetic loci in the ANDIS and the non-diabetic MDC-CVA cohorts revealed that no genetic variant previously associated with diabetes and/or related traits was associated with all clusters. The TCF7L2 variant was associated with SIDD, MOD and MARD, but not with SIRD, among other associations between gene variants and specific clusters.
Conclusion
In a data-driven cluster analysis of six variables in adult patients with newly diagnosed diabetes, five categories of patients were identified with different characteristics and different risks of diabetic complications. These results suggest that a more precise stratification of new onset diabetes is possible and clinically useful, and it can provide information about underlying disease mechanisms. This allows individualization of treatments according to risk of complications.
References
1. Tuomi T, Groop LC, Zimmet PZ, et al. Antibodies to glutamic acid decarboxylase reveal latent autoimmune diabetes mellitus in adults with a non-insulin-dependent onset of disease. Diabetes 1993; 42: 359–62.
2. Froguel P, Zouali H, Vionnet N, et al. Familial hyperglycemia due to mutations in glucokinase. Definition of a subtype of diabetes mellitus. N Engl J Med 1993; 328: 697–702.
3. Yamagata K, Oda N, Kaisaki PJ, et al. Mutations in the hepatocyte nuclear factor-1alpha gene in maturity-onset diabetes of the young (MODY3). Nature 1996; 384: 455–58.