NOAC reversal agent completely abolishes anticoagulation activity in elderly and renally impaired volunteers
Idarucizumab resulted in immediate, complete and sustained reversal of dabigatran anticoagulant activity, in middle-aged, elderly and renally impaired volunteers.
Effect of Age and Renal Function on Idarucizumab Pharmacokinetics and Idarucizumab-Mediated Reversal of Dabigatran Anticoagulant Activity in a Randomized, Double-Blind, Crossover Phase Ib StudyLiterature - Glund S et al., Clin Pharmacokinet 2016
Glund S,Stangier J, van Ryn J, et al.
Clin Pharmacokinet 2016; published online ahead of print
Background
Dabigatran is a direct thrombin inhibitor, indicated for the prevention and treatment of thromboembolic events in patients with atrial fibrillation (AF) [1–3]. Immediate reversal of the anticoagulation effect could be achieved with the intravenous use of idarucizumab in young and healthy volunteers [4-7].Idarucizumab is an antibody fragment that may be useful in elderly AF patients with renal dysfunction, or AF patients who must undergo urgent surgery, or have a major bleeding event, in which case it is important to re-start anticoagulation as soon as possible after reversal, in order to prevent stroke.
In this randomised, double-blind, cross-over study, the pharmacodynamics (PD) of the reversal of dabigatran anticoagulant (oral prodrug is dabigatran etexilate, DE) effect by idarucizumab was evaluated, in 46 middle-aged volunteers vs. healthy elderly, and mild (60-90) or moderately (30-60) renally impaired (RI) volunteers. All but the moderate RI group, received low or high doses which were variable among groups; 2.5 or 5 gr for middle-aged, 1 or 5 gr for elderly, 1 or 5 gr for 60-90 RI and 2x2.5 gr for 30-60 RI volunteers.
Main results
Dabigatran etexilate (DE):- After 2 hours, steady-state plasma levels of unbound dabigatran were 170 ng/mL in middle-aged volunteers vs. 140 and 220 ng/mL in volunteers with RI 60-90 respectively 30-60.
- Resulted in a 1.8-, 3-, 2.2-fold increase of diluted thrombin time (dTT), ecarin clotting time (ECT) and activated partial thromboplastin time (aPTT) in middle-aged patients.
- Resulted in a 3.1-, 3.6-, 2.2-fold increase of dTT, ECT, aPTT in elderly vs. middle-aged volunteers
- The idarucizumab plasma concentration–time profile in elderly volunteers was very similar to that of middle-aged volunteers.
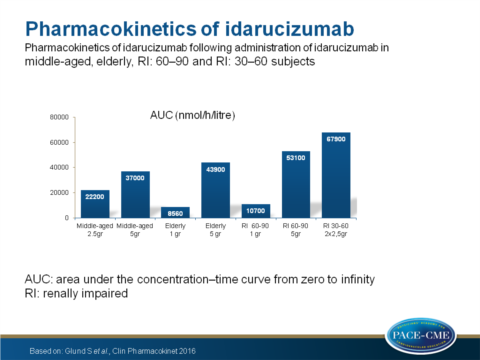
- Idarucizumab exposure (AUC) was similar in middle-aged and elderly patients but increased in 60-90 and 30-60 RI patients (P=0.002 and P<0.0001 respectively).
- Unbound dabigatran plasma concentrations decreased evenly between all groups <1ng/mL.
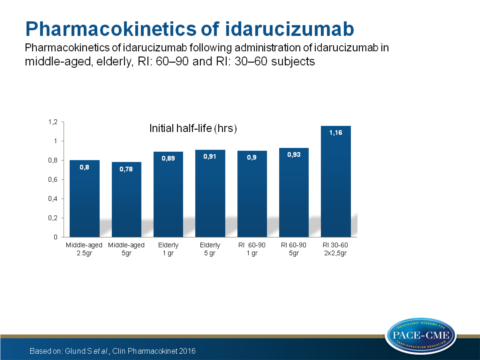
- The initial half time was equal between middle-aged and elderly volunteers but prolonged in both RI groups when compared with the middle-aged group P=0.0099 for 60-90 and P=0.0003 for 30-60).
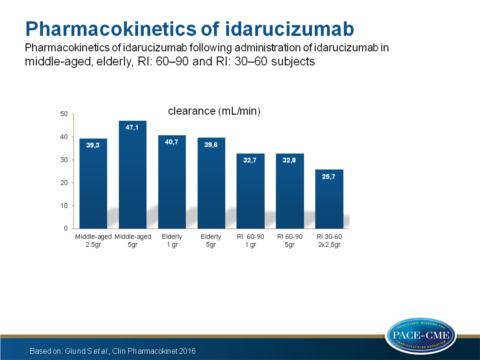
- Clearance was equal between middle-aged and elderly volunteers but decreased in 60-90 and 30-60 RI patients compared to the middle-aged group (P=0.002 and P<0.0001 respectively).
- The 1gr doses in elderly and 60-90 RI volunteers resulted in a partial return of dabigatran anticoagulation after 2 hours, which was not observed in middle-aged volunteers whom received 5 or 2.5 gr.
- All adverse events (46% for dabigatran, 30% for idarucizumab and 26% for placebo) were mild and independent of group.
Conclusion
Pharmacodynamic data showed that idarucizumab resulted in immediate, complete and sustained reversal of dabigatran anticoagulant activity independent of age or renal function. Impaired renal function was associated with increased exposure and decreased clearance of idarucizumab. Idarucizumab effects were dose-dependent. All doses were safe and well tolerated.Find this article online at Clinical Pharmacokinetics
References
1. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med.2009;361:1139–51.
2. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52.
3. Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129:764–72.
4. Sarich TC, Seltzer JH, Berkowitz SD, et al. Novel oral anticoagulants and reversal agents: considerations for clinical development. Am Heart J. 2015;169:751–7.
5. Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood.2013;121:3554–62.
6. Glund S, Stangier J, Schmohl M, et al. Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebocontrolled, double-blind phase 1 trial. Lancet. 2015;386:680–90.
7. Glund S, Moschetti V, Norris S, et al. A randomised study in healthy volunteers to investigate the safety, tolerability and pharmacokinetics of idarucizumab, a specific antidote to dabigatran. Thromb Haemost. 2015;113:943–51.
