Oral microsomal triglyceride transfer protein inhibitor is beneficial in HoFH
In patients with HoFH, lomitapide led to a significant reduction of LDL-c levels and to achievement of EAS targets in many patients, while CV event rates correlated with LDL-c levels.
Target achievement and cardiovascular event rates with Lomitapide in homozygous Familial HypercholesterolaemiaLiterature - Blom DJ, Cuchel M, Ager M, et al. - Orphanet J Rare Dis 2018 2018;13:96
Introduction and Methods
According to the European Atherosclerosis Society (EAS) recommendations, patients with homozygous familial hypercholesterolemia (HoFH) should be treated to the same LDL-c targets as other hypercholesterolemia patients, which, however, is not realistic in the majority of HoFH patients with currently available standard therapies [1]. Lomitapide, an oral microsomal triglyceride transfer protein inhibitor, has been approved as adjunctive therapy in adult patients with HoFH [2,3].
In this retrospective analysis, the number of HoFH patients receiving lomitapide who reach EAS targets (LDL-c level ≤70 mg/dL) was assessed. For this purpose, data from two lomitapide studies were used: a single-arm, open label, phase 3 clinical trial of lomitapide in adult patients with HoFH [2], and a single-arm extension of the study [3]. In both studies, the lomitapide dose was gradually increased from 5 mg to a maximum of 60 mg/day, and given on top of standard therapy until week 26. The primary endpoint was the mean percent change in LDL-c levels from baseline to week 26, after which patients remained on lomitapide until week 78 for safety assessment.
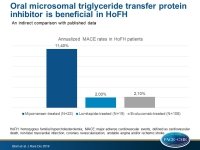
In addition, CV event rates in patients receiving lomitapide were compared with published data: the publication by Duell et al., including HoFH patients, who received mipomersen therapy for at least 12 months [4] and the publication by Raal et al., including a HoFH cohort treated with evolocumab [5].
MACE was defined as CV death, non-fatal myocardial infarction, coronary revascularization, unstable angina and/or ischemic stroke.
Main results
- In the phase 3 study (n=29), the baseline mean LDL-c was 336 ±114 mg/dL (8.70 ± 2.95 mmol/L), which was lowered by lomitapide treatment (median dose 40 mg) by a mean of 50.7% at week 26, and more than half of patients reached a target LDL-c level <100 mg/dL at least once by week 26.
- Moreover, 28% of patients reached the target of <70 mg/dL at least once by week 26, and 39% of those who remained in the study by week 78 reached this target as well.
- Out of 23 patients at the end of the phase 3 study, 19 entered the extension trial, and 16 completed end of all study assessments. The lomitapide LDL-c-lowering efficacy was maintained during the extension study (LDL-c lowering of 45.5% at week 126).
- 42% of patients in the extension trial reached the <70 mg/dL target at least once by week 126, and 58% reached the same target at least once by week 256.
- The MACE rate in the lomitapide trials was 1.7 events/1,000 months of treatment, which is equivalent to a 2.0% annualized event rate. In the mipomersen study the rate was 9.5 MACE events/1,000 months, equivalent to a 11.4% annualized event rate, and in the evolocumab trial the rate was 1.8 MACE events/1,000 months, equivalent to a 2.1% annualized event rate.
Conclusion
In patients with HoFH, lomitapide led to a significant reduction of LDL-c levels and to achievement of EAS targets in approximately half of lomitapide-treated patients after at least 2 years on treatment. CV event rates correlated with LDL-c levels.
References
1. Cuchel M, Bruckert E, Ginsberg HN, et al. European Atherosclerosis Society Consensus Panel on Familial Hypercholesterolaemia. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the consensus panel on familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014;35:2146–57.
2. Cuchel M, Meagher EA, du Toit Theron H, et al. Phase 3 Ho FHLSi. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381:40–6.
3. Blom DJ, Averna MR, Meagher EA, et al. Long-term efficacy and safety of the microsomal triglyceride transfer protein inhibitor Lomitapide in patients with homozygous familial hypercholesterolemia. Circulation. 2017;136:332–5.
4. Duell PB, Santos RD, Kirwan BA, et al. Long term mipomersen treatment is associated with a reduction in cardiovascular events in patients with familial hypercholesterolemia. J Clin Lipidol. 2016;10:1011–21.
5. Raal FJ, Hovingh GK, Blom D, et al. Long-term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in patients with homozygous familial hypercholesterolaemia: an interim subset analysis of the open-label TAUSSIG study. Lancet Diabetes Endocrinol. 2017