PCSK9 inhibition benefits patients with type 2 diabetes similar to non-diabetics
In 3 studies with data on patients with type 2 diabetes mellitus, the PCSK9 inhibitor evolocumab reduced levels of LDL-C, non-HDL-C, lipoprotein(a), and total cholesterol, and increased HDL levels.
Lipid-lowering efficacy of the PCSK9 inhibitor evolocumab (AMG 145) in patients with type 2 diabetes: a meta-analysis of individual patient dataLiterature - Sattar N et al., Lancet Diabetes Endocrinol 2016
Sattar N, Preiss D, Robinson JG, et al.
Lancet Diabetes Endocrinol 2016; published online ahead of print
Background
Patients with type 2 diabetes mellitus (T2DM) often have low HDL-c and high triglyceride-rich apolipoprotein B-carrying lipoproteins with only moderately raised LDL-c concentrations [1], and carry double the risk of myocardial infarction and stroke [2]. LDL-c lowering therapies like statins and ezetimibe are of benefit for these patients, as shown in relevant studies:- In the Collaborative Atorvastatin Diabetes Study, atorvastatin lowered LDL-c by 1·2 mmol/L, or about 40%, relative to placebo and led to a 37% reduction in the occurrence of major CV events over 3.9 years in patients with T2DM and no history of CV disease [3].
- In the Cholesterol Treatment Trialists’ Collaboration analysis of 14 major statin trials, patients with diabetes were at 9% lower risk of all-cause death and 21% lower risk of major CV events per 1 mmol/L reduction in LDL-c achieved with statin therapy compared with patients who not on statins [4].
- In a subgroup analysis of patients with diabetes who had a recent acute coronary syndrome in the IMPROVE-IT trial, the addition of ezetimibe to simvastatin led to a further 0.4 mmol/L lowering of LDL-c and to a 14% reduction in major CV events, compared with simvastatin alone [5].
Main results
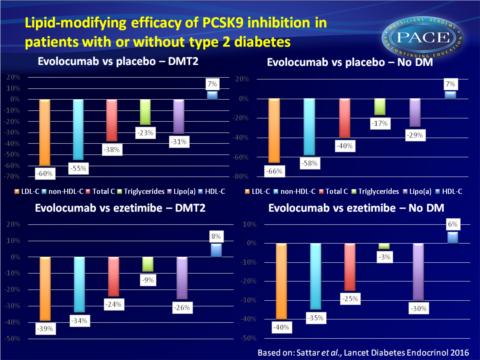
- Evolocumab versus placebo:- In patients with T2DM:
- mean reductions were seen: in LDL-c of 60%; 95%CI: 51–69, in non-HDL-C of 55%; 95%CI: 47–63, in total cholesterol of 38%; 95%CI: 32-44, in triglycerides of 23%; 95%CI: 12-34, and in lipoprotein(a) of 31%; 95%CI: 25–37.
- And a mean increase in HDL-c of 7%; 95%CI: 4–11 (P < 0.0001 for all comparisons). - In patients without T2DM
- mean reduction were seen: in LDL-c of 66%; 95%CI: 62–70, in non-HDL-C of 58%; 95%CI: 55-61, in total cholesterol of 40%; 95%CI: 37-42, in triglycerides of 17%; 95%CI: 14-21, and in lipoprotein(a) of 29%; 95%CI: 26–31.
- And a mean increase in HDL-c of 7%; 95%CI: 5-9 (P < 0.0001 for all comparisons).
- In patients with T2DM:
- mean reductions were seen in LDL-c of 39%; 95%CI: 32-47, in non-HDL-C of 34%; CI 95%CI: 26-41, in total cholesterol of 24%; 95%CI: 16-31, in triglycerides of 9%; 95%CI: 5-23, and in lipoprotein(a) of 26%; 95%CI: 16-35.
- And a mean increase in HDL-c of 8%; 95%CI: 4–13. P = 0.20 for triglycerides, P < 0.0001 for all other comparisons - In patients without T2DM:
- mean reduction were seen in LDL-c of 40%; 95%CI: 36-45, in non-HDL-C of 35%; 95%CI: 31-40, in total cholesterol of 25%; 95%CI: 23-27, in triglycerides of 3%; 95%CI: 3-8, and in lipoprotein(a) of 30%; 95%CI: 24–36.
- And a mean increase in HDL-c of 6%; 95%CI: 3-9 (P = 0.31 for triglycerides, P < 0.0001 for all other comparisons) - For all lipid parameters, no interaction was seen for treatment effect and having T2DM or not.
Download Sattar Lancet Diab Endocrino 2016_PACE.pptx
Conclusion
In patients with type 2 diabetes mellitus, the PCSK9 inhibitor evolocumab reduced the levels of LDL-C, non-HDL-C, lipoprotein(a), and total cholesterol, and increased HDL levels significantly, compared with placebo and with ezetimibe. These reductions were consistent with what was observed in patients without diabetes.Editorial comment [6]
‘This study extends previous evidence of the robust cholesterol-lowering effects of PCSK9 inhibitors to patients with type 2 diabetes. These effects were seen in patients who were already taking modern statin therapy, and evolocumab was superior to ezetimibe and well tolerated.’ However, caution is advised with regard to generalisation of the results of this meta-analysis, because:- the short duration of included studies restricts the proper assessment of adverse events
- it is not clear whether PCSK9 inhibitors could negatively affect glucose homeostasis
- the high cost of PCSK9 inhibitors initially limits their use to patients who are at the highest CV risk and who are not adequately managed with alternative lipid-lowering agents
Find this article online at The Lancet
References
1. Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 2004; 27: 1496–504
2. The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375: 2215–22
3. Colhoun HM, Betteridge DJ, Durrington PN, et al, on behalf of the CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomized placebo-controlled trial. Lancet 2004; 317: 117–25
4. Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy of cholesterol-lowering therapy in 18 686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371: 117–25
5. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372: 2387–97
6. Santos RD. PCSK9 inhibition in type 2 diabetes: so far so good, but not there yet. Lancet Diabetes Endocrinol 2016; published online ahead of print
