PCSK9 inhibition: hope for treating dyslipidemia?
J. Lipid research, July 2012 . A lot of research has been done to understand the biology of PCSK9. This article describes the role of PSCK9 in cholesterol metabolism and data on PCSK9 inhibition: A summary.
The PSCK9 decadeLiterature - Lambert G et al. J Lipid Res. 2012 Jul 17
PCSK9 inhibition: hope for treating dyslipidemia?
Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK.
J Lipid Res. 2012 Jul 17. [Epub]
Background
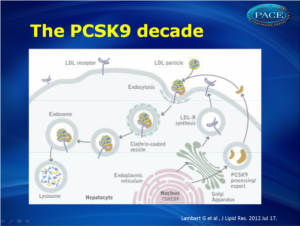
Proprotein convertase subtilisin kexin type 9 (PCSK9) plays a pivotal role in LDL metabolism as it promotes the degradation of the LDL receptor and prevents it from recycling to the membrane. PCSK9 is therefore a novel target for lipid-lowering therapy. Inhibition of PCSK9 acts synergistically with existing treatments such as statins. A lot of research has been done to understand the biology of PCSK9. This article describes the role of PSCK9 in cholesterol metabolism and data on PCSK9 inhibition.
Main results
PCSK9 inhibition is an interesting target to further lower LDL-C levels. The mechanism of action is shown in figure 1.
Conclusion
PSCK9 inhibition is efficacious in reducing LDL-C levels. It has to be further evaluated whether this also leads to reduction in coronary risk.References
- McKenney, J., M. Koren, D. Kereiakes, et al. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J. Am. Coll. Cardiol. [Epub ahead of print]
- Stein EA, Gipe D, Bergeron J, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce lowdensity lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. May 25 [Epub ahead of print]
- Stein, E. A., S. Mellis, G. D. Yancopoulos, et al. Effect of a monoclonal antibody to PCSK9 on LDL Cholesterol. N. Engl. J.Med. 366: 1108-1118.