PCSK9 inhibition to reach LDL targets
05/04/2012
PCSK9 inhibition on top of atorvastatin therapy, lowers LDL-cholesterol up to 72%, and ApoB in patients with primary hypercholesterolemia.
Safety and efficacy of a monoclonal antibody to PCSK9, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy.Literature - McKenny JM et al, J Am Coll Cardiol. 2012 Mar 28.
McKenny JM, Koren MJ, Kereiakes DJ et al.
J Am Coll Cardiol. 2012 Mar 28.
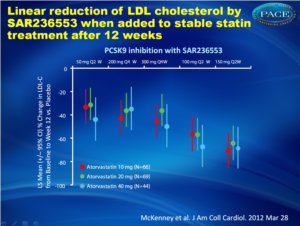
Specific binding of PCSK9 with an antibody in a study showed clear dose-dependent additional lowering of LDL-cholesterol levels in patients with primary hypercholesterolemia receiving a stable dose of 10, 20 or 40 mg per day of atorvastatin.
McKenny JM, Koren MJ, Kereiakes DJ et al. JACC expedited publication (doi:10.1016/j.jacc2012.03.007)
Serum proprotein convertase subtilisin kexin 9 (PCSK9) binds to low-density-lipoprotein (LDL)-receptors, reduce LDL-receptor uptake and thereby increase LDL-cholesterol concentration1. In trials of nature both increased and decreased functioning of PCSK9 have been described leading to subsequent changes in LDL levels. SAR236553 is a human monoclonal antibody that specifically binds PCSK9, leading to lowering of LDL-cholesterol levels.
In patients with primary hypercholesterolemia monotherapy with statin treatment effectively lowers (LDL)-cholesterol levels, but in many patients target levels of LDL-cholesterol will not be achieved. New approaches are being studied to reach superior LDL-lowering 2. Combining different classes of products is needed to lower LDL-cholesterol levels further to reduce build up of atherosclerotic plaques and prevent events3. Lower (LDL-) cholesterol levels reduce event rates more4. In this double-blind, placebo-controlled, type two dose-finding study, the addition of the PCSK9-antibody effectively reduced the LDL-cholesterol and ApoB concentrations. The main focus is on efficacy and safety profile in a twelve week dosing schedule.
There were six parallel patients groups receiving placebo or SAR236553 50, 100 or 150 mg dose, every two weeks, and another two groups of patients received 200 and 300 mg dose SAR236553 every four weeks with placebo injections in- between. Each patient group received biweekly injections for 12 weeks, effectively blinding doctors and patients.
LDL was lowered in an effective and near linear fashion.
SAR236533 was generally well tolerated; there was only one serious adverse event. Inhibiting PCSK9 can substantially further reduce LDL-cholesterol to very low levels. At a dose of 100 mg every other week most of patients reached LDL and ApoB target levels. At the maximum dose of 150 mg every other week, LDL –cholesterol was below 70 mg/dl (or < 0,8 mmol/l), non-HDL cholesterol was below 100mg/dl (1,13 mmol/l) and ApoB was reduced to less than 0,8 mmol/l.
There seems to be no attenuation of the effect after the three months, there may be a trend towards further efficacy of SAR236553 at the end of the three month period in all three biweekly dosing regimens.
Another observation is of course that dosing the antibody monthly results in a rebound of LDL at the end of the treatment interval, the effect seriously wanes off to 50% of the initial reduction. Extended use of SAR236553 is ongoing to establish the clinical effects of these promising short term lipid changes.
SAR263533 holds a very interesting promise for all patients who after taking even combined high doses of cholesterol lowering medication still do not reach target levels of LDL- and non-HDL-cholesterol.
2. Wierzbicki, A. S., Hardman, T. C. and Viljoen, A. (2012), New lipid-lowering drugs: an update. International Journal of Clinical Practice, 66: 270–280. doi: 10.1111/j.1742-1241.2011.02867.x
3. Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769-1818
4. Hsia J, MacFayden JG, Monyak J, Ridker PM. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol 50 mg/dl with rosuvastatin. The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). J Am Coll Cardiol 2011;57:1666 –75.
Background Serum proprotein convertase subtilisin kexin 9 (PCSK9) binds to low-density lipoprotein receptors, increasing serum LDL-C. SAR236553 is a fully human monoclonal antibody to PCSK9.
Methods This double-blind, parallel-group, placebo-controlled trial randomized 183 patients with LDL-C ≥ 100 mg/dl (2.59 mmol/l) on stable-dose atorvastatin 10, 20, or 40 mg for ≥ 6 weeks to subcutaneous placebo every 2 weeks (Q2W); SAR236553 50, 100, or 150 mg Q2W; or SAR236553 200 or 300 mg every 4 weeks (Q4W), alternating with placebo for a total treatment period of 12 weeks.
Results SAR236553 demonstrated a clear dose-response relationship with respect to percentage LDL-C lowering for both Q2W and Q4W administration: 40%, 64%, and 72% with 50, 100, and 150 mg Q2W, respectively, and 43% and 48% with 200 and 300 mg Q4W. LDL-C reduction with placebo at week 12 was 5%. SAR236553 also substantially reduced non–high-dose lipoprotein cholesterol, apolipoprotein B, and lipoprotein(a). SAR236553 was generally well tolerated.
One patient on SAR236553 experienced a serious adverse event of leukocytoclastic vasculitis.
Conclusions When added to atorvastatin, PCSK9 inhibition with SAR236553 further reduces LDL-C by 40% to 72%. These additional reductions are both dose- and dosing frequency–dependent.
J Am Coll Cardiol. 2012 Mar 28.
Specific binding of PCSK9 with an antibody in a study showed clear dose-dependent additional lowering of LDL-cholesterol levels in patients with primary hypercholesterolemia receiving a stable dose of 10, 20 or 40 mg per day of atorvastatin.
McKenny JM, Koren MJ, Kereiakes DJ et al. JACC expedited publication (doi:10.1016/j.jacc2012.03.007)
Background
Serum proprotein convertase subtilisin kexin 9 (PCSK9) binds to low-density-lipoprotein (LDL)-receptors, reduce LDL-receptor uptake and thereby increase LDL-cholesterol concentration1. In trials of nature both increased and decreased functioning of PCSK9 have been described leading to subsequent changes in LDL levels. SAR236553 is a human monoclonal antibody that specifically binds PCSK9, leading to lowering of LDL-cholesterol levels.In patients with primary hypercholesterolemia monotherapy with statin treatment effectively lowers (LDL)-cholesterol levels, but in many patients target levels of LDL-cholesterol will not be achieved. New approaches are being studied to reach superior LDL-lowering 2. Combining different classes of products is needed to lower LDL-cholesterol levels further to reduce build up of atherosclerotic plaques and prevent events3. Lower (LDL-) cholesterol levels reduce event rates more4. In this double-blind, placebo-controlled, type two dose-finding study, the addition of the PCSK9-antibody effectively reduced the LDL-cholesterol and ApoB concentrations. The main focus is on efficacy and safety profile in a twelve week dosing schedule.
Main results
There were six parallel patients groups receiving placebo or SAR236553 50, 100 or 150 mg dose, every two weeks, and another two groups of patients received 200 and 300 mg dose SAR236553 every four weeks with placebo injections in- between. Each patient group received biweekly injections for 12 weeks, effectively blinding doctors and patients.LDL was lowered in an effective and near linear fashion.
SAR236533 was generally well tolerated; there was only one serious adverse event. Inhibiting PCSK9 can substantially further reduce LDL-cholesterol to very low levels. At a dose of 100 mg every other week most of patients reached LDL and ApoB target levels. At the maximum dose of 150 mg every other week, LDL –cholesterol was below 70 mg/dl (or < 0,8 mmol/l), non-HDL cholesterol was below 100mg/dl (1,13 mmol/l) and ApoB was reduced to less than 0,8 mmol/l.
There seems to be no attenuation of the effect after the three months, there may be a trend towards further efficacy of SAR236553 at the end of the three month period in all three biweekly dosing regimens.
Another observation is of course that dosing the antibody monthly results in a rebound of LDL at the end of the treatment interval, the effect seriously wanes off to 50% of the initial reduction. Extended use of SAR236553 is ongoing to establish the clinical effects of these promising short term lipid changes.
Conclusion
SAR263533 holds a very interesting promise for all patients who after taking even combined high doses of cholesterol lowering medication still do not reach target levels of LDL- and non-HDL-cholesterol.
References
1. Abifadel M, Varret M, Rabès JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003;34:154–6.2. Wierzbicki, A. S., Hardman, T. C. and Viljoen, A. (2012), New lipid-lowering drugs: an update. International Journal of Clinical Practice, 66: 270–280. doi: 10.1111/j.1742-1241.2011.02867.x
3. Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769-1818
4. Hsia J, MacFayden JG, Monyak J, Ridker PM. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol 50 mg/dl with rosuvastatin. The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). J Am Coll Cardiol 2011;57:1666 –75.
Abstract
Objectives The primary objective of this study was to evaluate the low-density lipoprotein cholesterol (LDL-C)–lowering efficacy of 5 REGN727/SAR236553 (SAR236553) dosing regimens versus placebo at week 12 in patients with LDL-C ≥ 100 mg/dl on stable atorvastatin therapy. Secondary objectives included evaluation of effects on other lipid parameters and the attainment of LDL-C treatment goals of <100 mg/dl (2.59 mmol/l) and < 70 mg/dl (1.81 mmol/l).Background Serum proprotein convertase subtilisin kexin 9 (PCSK9) binds to low-density lipoprotein receptors, increasing serum LDL-C. SAR236553 is a fully human monoclonal antibody to PCSK9.
Methods This double-blind, parallel-group, placebo-controlled trial randomized 183 patients with LDL-C ≥ 100 mg/dl (2.59 mmol/l) on stable-dose atorvastatin 10, 20, or 40 mg for ≥ 6 weeks to subcutaneous placebo every 2 weeks (Q2W); SAR236553 50, 100, or 150 mg Q2W; or SAR236553 200 or 300 mg every 4 weeks (Q4W), alternating with placebo for a total treatment period of 12 weeks.
Results SAR236553 demonstrated a clear dose-response relationship with respect to percentage LDL-C lowering for both Q2W and Q4W administration: 40%, 64%, and 72% with 50, 100, and 150 mg Q2W, respectively, and 43% and 48% with 200 and 300 mg Q4W. LDL-C reduction with placebo at week 12 was 5%. SAR236553 also substantially reduced non–high-dose lipoprotein cholesterol, apolipoprotein B, and lipoprotein(a). SAR236553 was generally well tolerated.
One patient on SAR236553 experienced a serious adverse event of leukocytoclastic vasculitis.
Conclusions When added to atorvastatin, PCSK9 inhibition with SAR236553 further reduces LDL-C by 40% to 72%. These additional reductions are both dose- and dosing frequency–dependent.