PCSK9 inhibitor also lowers Lp(a) in hypercholesterolemia
Lp(a) showed a dose-response relationship for both dosing frequencies, and Lp(a) reduction was consistent across several patient subgroups.
AMG 145, A Monoclonal Antibody Against PCSK9, Significantly Reduces Lipoprotein (a) in Hypercholesterolemic Patients Receiving Statin Therapy: An Analysis From the LAPLACE-TIMI 57 Trial.Literature - Desai NR, Kohli P, Giugliano RP et al. - Circulation. 2013 Jul 24
Desai NR, Kohli P, Giugliano RP et al.
Circulation. 2013 Jul 24
Background
Lipoprotein (a) (Lp(a)) consists of an apolipoprotein B100 (ApoB) molecule bound to a liver-derived glycoprotein apolipoprotein (a) (Apo(a)) [1]. Lp(a) is thought to play a role in tissue healing and innate immunity, but its exact function remains unclear, although it is now recognised for its proatherosclerotic and prothrombotic effects [2]. Lp(a) can promote smooth muscle cell proliferation, endothelial cell adhesion molecule expression, foam cell generation and endothelial dysfunction, and it can act as a competitive inhibitor for plasminogen and it has antifibrinolytic effects.Lp(a) has now emerged as an important risk factor for cardiovascular disease (CVD) [3-5], and the European Society of Atherosclerosis recommends assessment of Lp(a) in individuals with premature CVD, familial hypercholesterolaemia, or a family history of premature CVD and/or elevated Lp(a), and/or with recurrent CVD despite statin therapy [6].
Only limited therapeutic options exist for lowering Lp(a) levels. AMG 145 is a fully human monoclonal antibody to PCSK9, a protein synthesised and secreted by hepatocytes that binds to the LDL-receptor, thereby marking it for lysosomal degradation. AMG 145 gave significant, dose-dependent LDL-c reductions in patients with hypercholesterolemia receiving statin therapy with or without ezetimibe, in the LDL-C Assessment with PCSK9Monoclonal Antibody Inhibition Combined With Statin Therapy (LAPLACE)-TIMI 57 trial [7]. This study investigated the effect of PCSK9 inhibition with AMG 145 on Lp(a) in this patient population.
Main results
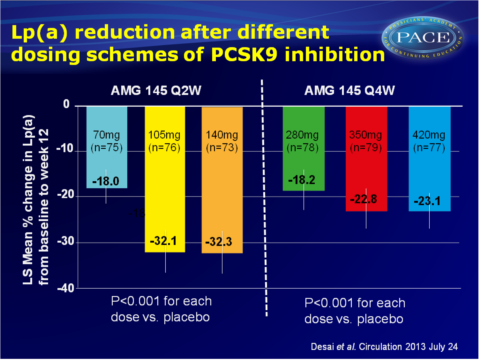
- All doses of AMG 145 significantly reduced Lp(a) levels from baseline to week 12, as compared to placebo (P<0.001 for all doses). Mean percentages reduction in Lp(a) were 18.0%, 32.1% and 32.3% for subjects receiving AMG 145 70 mg, 105 mg and 140 mg every 2 weeks respectively, and 18.2%, 22.8% and 23.1% when AMG 145 was given at 280 mg, 350 mg and 420 mg every 4 weeks.
- Only a moderate positive correlation was observed between percentage change in Lp(a) and the percentage change in LDL-c (ρ=0.33, P<0.001) or apoB (ρ=0.36, P<0.001). A weak correlation between percentage change in Lp(a) and PCSK9 was seen (ρ=0.12, P<0.013).
- Percentage change in Lp(a) was consistent irrespective of age, sex, race, history of diabetes, intensity of background statin therapy and LDL-c at study entry for AMG 145 140 mg every 2 weeks and 420 mg every 4 weeks.
- Smaller percentage reductions in Lp(a) were seen across progressively higher quartiles of baseline Lp(a) for AMG 145 140 mg every 2 weeks and 420 mg every 4 week, as compared to placebo. Absolute reductions in Lp(a), on the other hand, were larger in higher quartiles of baseline Lp(a) for these doses.
Download Desai AMG 145_ PACE.pptxor click to enlarge.
Conclusion
AMG 145 significantly lowered Lp(a) in subjects with hypercholesterolemia on background lipid-lowering therapy, in a dose-dependent manner, at both dosing frequencies. A plateau effect appears to occur, which is similar to the biology of PCSK9 inhibition with AMG 145. The reduction in Lp(a) was found to be consistent across several clinically relevant subgroups. Baseline Lp(a) affected the percentage Lp(a) response to AMG 145.The precise mechanism via which AMG 145 lowers Lp(a) remains to be elucidated. The reduction of Lp(a) with AMG 145 represents an additional benefit, complementary to its LDL-c-lowering effects.
References
1. Kathiresan S. Lp(a) lipoprotein redux--from curious molecule to causal risk factor. N Engl J Med. 2009;361:2573-2574.
2. Kiechl S, Willeit J. The mysteries of lipoprotein(a) and cardiovascular disease revisited. J Am Coll Cardiol. 2010;55:2168-2170.
3. Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412-423.
4. Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331-2339.
5. Clarke R, Peden JF, Hopewell JCet al. Genetic variants associated with lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518-2528.
6. Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: Current status. Eur Heart J. 2010;31:2844-2853.
7. Giugliano RP, Desai NR, Kohli P, Rogers et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (laplace-timi 57): A randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380:2007-2017.
Find this article on Pubmed
