PCSK9-inhibitor yields greater LDL-c reduction than conventional intensive lipid-lowering strategies
PCSK9-inhibitor alirocumab as add-on to statin therapy lowers LDL-c levels more than adding ezetimibe or increasing the dose or intensity of statin treatment in ODYSSEY OPTIONS trial.
Alirocumab as Add-on To Atorvastatin Versus Other Lipid Treatment Strategies: ODYSSEY OPTIONS I Randomized TrialLiterature - Bays H et al., J Clin Endocrinol Metab. 2015
Bays H, Gaudet D, Weiss R et al.,
J Clin Endocrinol Metab. 2015 Jun 1:jc20151520. [Epub ahead of print]
Background
Despite receiving standard-of-care therapy, most patients with coronary heart disease (CHD) still do not reach LDL-c target [1]. Clinical outcome data suggest that further lowering of the LDL-c level may reduce the risk of CHD [2,3].Strategies that have been shown to give additional LDL-c reductions include doubling the statin dose, switching to a higher-potency statin, and adding a nonstatin lipid-lowering drug.
Alirocumab is an investigational monoclonal antibody directed against proprotein convertase subtilisin/kexin type 9 (PCSK9), that has been shown to reduce LDL-c levels by 40% to 70% when given as monotherapy, or in addition to statin therapy [4-7].
The ODYSSEY OPTIONS I trial directly compared the efficacy and safety of alirocumab in addition to atorvastatin, with other lipid-lowering strategies, namely A: addition of ezetimibe, B: doubling atorvastatin dose, or C: switching from atorvastatin 40 mg to rosuvastatin 40 mg. OPTIONS I was a multicentre, randomised, double-blind, double-dummy 24-week phase III trial in 355 patients with hypercholesterolaemia at high or very-high cardiovascular disease risk, on a stable dose of atorvastatin 20 mg or 40 mg.
Main results
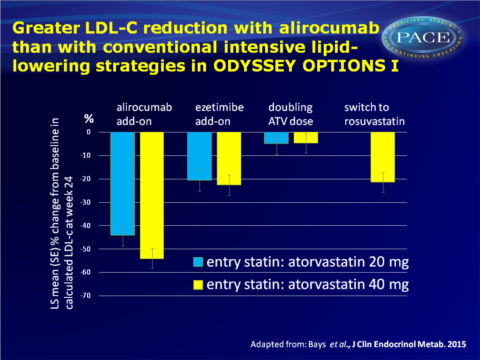
- Add-on alirocumab reduced LDL-c levels at 24 weeks by 44.1% and 54.0% in the atorvastatin 20 and 40 mg regimens respectively, while add-on ezetimibe lowered LDL-c by 20.5% and 22.6%, doubling of atorvastatin by 5.0% and 4.8% and switching from atorvastatin 40 mg to rosuvastatin further lowered LDL-c by 21.4%.
- LDL-c reductions were stable from week 4 through week 24.
- Most (86%) patients treated with alirocumab add-on were maintained on the 75 mg Q2W dose after week 12, because they reached the predefined threshold of <70 or 100 mg/dL at week 8. 8.0% and 20.9% of patients on baseline atorvastatin 20 mg and 40 mg respectively, switched to 150 mg Q2W at week 12.
- Over 80% of high and very-high CVD risk patients achieved their protocol defined LDL-c goals of <70 and <100 mg/dL respectively with add-on alirocumab treatment (calculated LDL-c values).
- The rates of treatment emergent adverse events (TEAEs), serious AEs and injection site reactions were comparable among all treatment regimes.
- 5 of 99 patients (5.1%) receiving alirocumab add-on therapy showed treatment-emergent antidrug antibody responses, 3 of which were persistent.
Download Bays ODYSSEY OPTIONS 1 2015 PACE.pptx
Conclusion
In patients at high CVD risk who are treated with atorvastatin 20 or 40 mg, adding alirocumab stably provided greater LDL-c reductions than adding ezetimibe or increasing the dose or intensity of the statin treatment. The impact of the further LDL-c lowering with alirocumab on CV events will be assessed in the ongoing ODYSSEY OUTCOMES study.Find this article online at the J Clin Endocrinol Metab
References
1. Hess G, Chang CL, Chung K. Lipid attainment among patients newly treated with lipid-altering drugs. Curr Med Res Opin. 2014;30:1743–1756.
2. Baigent C, Blackwell L, Emberson J et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet.
2010;376:1670–1681.
3. Merck Sharp, Dohme Corp. Merck Provides Update on IMPROVE-IT Trial. 2014. Available at: http://www.mercknewsroom.com/press-release/research-and-development-news/merckprovides-
update-improve-it-trial.
4. McKenney JM, Koren MJ, Kereiakes DJ, et al. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–2353.
5. Roth EM, McKenney JM, Hanotin C, et al. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367:1891–1900.
6. Stein EA, Gipe D, Bergeron J, et al. Effect of a monoclonal antibody to PCSK9, REGN727/
SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised
controlled trial. Lancet. 2012;380:29–36.
7. Roth EM, Taskinen MR, Ginsberg HN, et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized Phase 3 trial. Int J Cardiol. 2014;176:55–61.
