Pioglitazone/alogliptin combination therapy improves islet-cell function in T2DM
By addressing multiple core defects of T2DM at once, ALO/PIO combination therapy can safely improve fasting and postprandial glucose levels.
The effect of alogliptin and pioglitazone combination therapy on various aspects of β-cell function in patients with recent-onset type 2 diabetesLiterature - Van Raalte et al., Eur J Endocrinol. 2014 - Eur J Endocrinol. 2014 Mar 8;170(4):565-74
Van Raalte DH, van Genugten RE, Eliasson B et al.
Eur J Endocrinol. 2014 Mar 8;170(4):565-74.
Background
In type 2 diabetes mellitus (T2DM), β-cell function is impaired, in the context of reduced insulin sensitivity [1,2]. Progressive β-cell dysfunction may explain why optimal glycaemic control is often only achieved for a short duration [3]. The currently advocated stepwise treatment paradigm may also contribute to this process [4]. The stepwise approach may be ineffective, for instance because intensification is often delayed [5]. Also, current treatment strategies does not address all defects that are simultaneously at play in T2DM, such as islet-cell dysfunction, insulin resistance and impaired incretin action.An alternative therapeutic approach is to start aggressively, by using combinations of agents that address multiple issues in T2DM simultaneously. Thus far, evidence for this strategy is scarce [1,5,6].
A combination of dipeptidyl peptidase-4 (DPP4-inhibitors) and thiazolidinediones (TZDs) has been shown to improve glycaemic control in T2DM, both as add-on treatment and as initial combination therapy. The initial effects of combined DPP4/TZD therapy on β-cell function in T2DM patients have not been studied.
This 16-week phase IIIB randomised, double-blind a study therefore investigated the effects of initial combination of the DPP4-inhibitor alogliptin (ALO) and the TZD pioglitazone (PIO) on β-cell function, in T2DM patients whose glycaemic control was just off target on monotherapy with one single oral hypoglycaemic agent, in comparison with ALO-monotherapy or placebo. Pancreatic β-cell function was assessed using a model that describes the relationship between insulin secretion and glucose concentration [7,8]
Main results
- After 16 weeks of treatment, both ALO/PIO and ALO significantly reduced A1c from baseline, as compared with placebo (-0.4+0.2 mmol/L for ALO and -0.9+0.1 mmol/L for ALO/PIO, both P<0.001). Similarly, fasting glucose levels were significantly decreased by both ALO/PIO and ALO treatment, with a stronger effect for combination therapy than ALO monotherapy.
- Postprandial glucose concentrations were reduced after ALO/PIO and ALO therapy as compared with placebo, with ALO/PIO (AUC: -22+4) giving a significantly larger decrease than ALO (AUC: -12+6). ALO/PIO treatment also reduced postprandial insulin and C-peptide concentration as compared to placebo, while ALO monotherapy did not.
- ALO/PIO treatment improved fasting insulin secretion at a fixed glucose level (35+19%), as well as β-cell glucose sensitivity (58+18%), while ALO monotherapy did not. Insulogenic index and rate sensitivity as measures for early insulin secretion were not significantly altered as a result of any of the therapies.
- Fasting insulin sensitivity (HOMA-IR) was significantly increased by combination therapy as compared with ALO monotherapy and placebo. ALO monotherapy did not alter HOMA-IR. Postprandial insulin sensitivity (OGIS) was improved by both combination and ALO monotherapy, although to a larger extent in ALO/PIO.
- Adverse events were seen in 59% of patients in the ALO/PIO group, in 63% of the placebo-users and 76% of the ALO group. Most AEs were mild and assess to be related to study treatment.
Download Raalte 2014 PACE.pptx
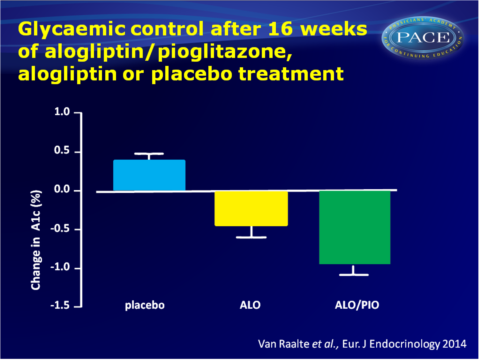
Conclusion
This study shows for the first time that pioglitazone/alogliptin combination therapy improves islet-cell function as compared with ALO monotherapy and placebo, in uncomplicated T2DM patients who are just above glycaemic targets on a single oral antihyperglycaemic agent. Insulin sensitivity improved with ALO/PIO combination therapy, as well as glycaemic control, and to a larger extent than ALO monotherapy. Combination therapy was well tolerated.Thus, by simultaneously addressing multiple core defects of T2DM, ALO/PIO combination therapy can safely improve fasting and postprandial glucose levels. Further studies need to evaluate possible benefits of early aggressive combination therapy, as compared with the currently advocated stepwise approach.
Find this article on Pubmed
References
1 Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009 58 773–795.
2 Weyer C, Bogardus C, Mott DM & Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. Journal of Clinical Investigation 1999 104 787–794.
3 Saydah SH, Fradkin J & Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. Journal of the American Medical Association 2004 291 335–342.
4 Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the
European Association for the Study of Diabetes (EASD). Diabetologia 2012 55 1577–1596.
5 Zinman B. Initial combination therapy for type 2 diabetes mellitus: is it ready for prime time? American Journal of Medicine 2011 124 S19–S34.
6 Zinman B, Harris SB, Neuman J, et al. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet 2010 376 103–111.
7 Mari A, Schmitz O, Gastaldelli A, et al. Meal and oral glucose tests for assessment of b-cell
function: modeling analysis in normal subjects. American Journal of Physiology. Endocrinology and Metabolism 2002 283 E1159–E1166.
8 Mari A, Tura A, Gastaldelli A & Ferrannini E. Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes 2002 51 (Suppl 1) S221–S226.
