Prevalence of homozygous hypercholesterolaemia in the Netherlands higher than anticipated
Substantial phenotypic variability seen among patients with molecularly diagnosed homozygous autosomal dominant hypercholesterolaemia, and the majority does not meet phenotypic diagnostic criteria.
Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype-phenotype relationship, and clinical outcomeLiterature - Sjouke B et al. Eur Heart J. 2014 - Eur Heart J. 2014 Feb 28
Sjouke B, Kusters DM, Kindt I, et al.
Eur Heart J. 2014 Feb 28. [Epub ahead of print]
Background
Autosomal dominant hypercholesterolaemia (ADH) may be caused by mutations in the genes encoding the LDL-receptor (LDLR), apolipoprotein B (APOB) or PCSK9. Homozygous ADH (hoADH) can be the result of either homozygosity or compound heterozygosity. hoADH is characterised by increased levels of LDL-c and physical signs of cholesterol deposits in the skin, eyes and/or tendons. The lifelong exposure to elevated LDL-c levels typically leads to cardiovascular disease (CVD) at a young age in hoADH patients [1,2]. Statins importantly lower LDL-c levels, but additional therapy, e.g. LDL-apheresis is often required in these patients [3].The exact prevalence of hoADH is unclear, partly because previous estimates were mostly based on clinical, instead of molecular criteria [4]. Since the 1990s, a cascade screening programme has been exploited in the Netherlands to identify all ADH patients. The widespread awareness of this nationwide programme among general practitioners and medical specialists allowed for a study of the prevalence and clinical phenotype of molecularly defined hoADH in the Netherlands.
Main results
- 104682 individuals were screened for ADH mutations. 49 patients were identified to have pathogenic mutations; 20 homozygotes (hoFH) and 25 compound heterozygotes (compHeFH) for mutations in LDLR, and 4 homozygotes for APOB mutations (hoFDB). Four hoFH patients from two different families had consanguineous parents.
- Considering a population of 16722387 inhabitants, the prevalence of hoFH and compHeFH ranges from 1 in 371608 (95%CI: 1: 287356 to 1: 526316) to 1 in 407863 (95%CI: 1:312500 to 1: 588235) persons in The Netherlands (after exclusion of offspring of consanguineous parents). The prevalence of heterozygous FH (heFH) was estimated to be 1 in 319 persons.
- The prevalence of hoFDB is 1 in 4180597 (95%CI: 2109705 to 1: 209205021), and heterozygous FDB is estimated to be 1 in 1023 persons.
- Based on the calculated prevalences, there are 68636 heterozygous ADH patients in The Netherlands, yielding a heterozygous ADH prevalence of 1 in 244 individuals (1/319 + 1/1023).
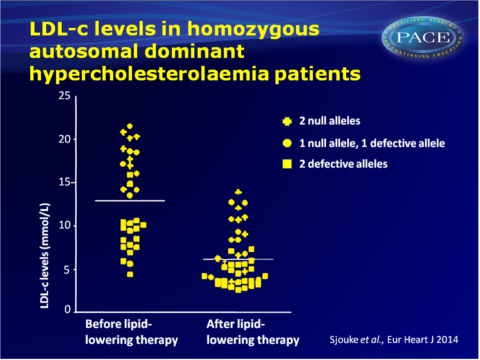
- Type of mutation significantly impacted LDL-c levels in statin-naïve patients: higher LDL-c levels were seen in patients with one or two null alleles, as compared with patients without null alleles (17.7+2.6 vs. 9.1+2.9 mmol/L; P<0.001).
- 49% of patients had LDL-c < 13.0 mmol/L, thus did not meet clinical criteria for hoADH. About 76% of patients did not have LDL-c>7.8 mmol/L while on lipid-lowering therapy.
- 30% of hoADH patients experienced a CV event, at an average age of onset of 34.2 +17.1 years (range: 13-69 years).
- All patients of whom information on therapy was available, were on lipid-lowering therapy. No patient reached target LDL-c as recommended by the current ESC/EAS guideline (<2.5 mmol/L).
Download Sjouke EHJ 2014 PACE.pptx
Conclusion
The prevalence of molecularly defined hoADH (hoFH and compHeFH and hoFDB) in the Netherlands was established to be ˜1 in 300000, which is at least three times more frequent than previously estimated. This study also showed a substantial phenotypic variation among patients molecularly diagnosed with hoADH, and importantly, a large part of patients did not fulfil the phenotypic criteria for hoADH. Molecular diagnosis of hoADH is important, since both parents and children of the hoADH patient will be heterozygous carriers. The current numbers suggest that only about one third of heterozygous ADH cases have been identified thus far.Find this article on Pubmed
References
1. Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolemia. In: Scriver C, Beaudet A, Sly W, Valle D (eds), The Metabolic and Molecular Bases of Inherited Disease. 8th ed. New York: McGraw-Hill; 2001. p. 2863 – 2913.
2. Kolansky DM, Cuchel M, Clark BJ,et al. Longitudinal evaluation and assessment of cardiovascular disease in patients with homozygous familial hypercholesterolemia. Am J Cardiol 2008;102:1438 – 1443.
3. Catapano AL, Reiner Z, DeBacker G, et al. ESC/EAS Guidelines for the management of dyslipidaemias The Task Force for the management of dyslipidaemias of the Euro- pean Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis 2011;217:3–46.
4. Raal FJ, Santos RD. Homozygous familial hypercholesterolemia: current perspectives on diagnosis and treatment. Atherosclerosis 2012;223:262 – 268.
