Promising results with PCSK9 siRNA treatment
Rapid and durable decrease in PCSK9 and LDL-c levels and encouraging safety profile with first-in-man siRNA targeted at PCSK9 in phase I trial.
Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trialLiterature - Fitzgerald et al., Lancet, Oct. 2013 - The Lancet, Early Online Publication, 3 October 2013
Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S et al.
The Lancet, Early Online Publication, 3 October 2013 doi:10.1016/S0140-6736(13)61914-5
Background
Despite the extensive use and efficacy of statins, the management of raised LDL-c remains inadequate, specifically in individuals with pre-existing coronary heart disease or diabetes, who are at high risk. It has been estimated that among high-risk individuals, only 50% achieve the target LDL-c of less than 2.59 mmol/L at 6 months after statin treatment, despite close monitoring en optimisation of the drug regimen [1-5]. Thus, the need for new treatments for hypercholesterolaemia is evident.Proprotein convertase subtilisin/kexin type 9 (PCSK9) has been identified as a potential therapeutic target, since experimental [6-8] and genetic studies [9-11] suggest that lowering circulating plasma PCSK9 levels should lower LDL-c, and possibly the risk of coronary heart disease. In addition, statins appear to increase circulating PCSK9 levels, which could limit the efficacy of statin treatment [12-15].
Small interfering RNA (siRNA) can cause sequence-specific degradation of messenger RNA, which suppresses the synthesis of the corresponding proteins. This is part of the naturally occurring RNA interference (RNAi) process [16,17]. A PCSK9-specific siRNA has been found to acutely lower hepatocyte-specific synthesis and plasma concentrations of PCSK9 in preclinical models [18], resulting in substantial and durable lowering of LDL-c.
This study investigated the safety and efficacy in human beings of intra-venous administration of ALN-PCS, an siRNA that inhibits PCSk9 synthesis formulated in a novel lipid nanoparticle for delivery [34], in a randomised, single-blind, placebo-controlled, phase I trial.
Main results
- ALN-PCS was generally safe and well-tolerated; no drug-related serious adverse events occurred. All treatment-emergent adverse events had mild to moderate severity, with similar proportions of patients in the ALN-PCS and placebo groups affected (19 (79%) vs. 7 (88%)). Both patients on ALN-PCS and placebo developed a mild, macular, transient erythematous rash, without pain or pruritus.
- No clinically significant, dose-dependent changes in laboratory indices (including liver function tests, creatine phosphokinase, C-reactive protein, haematologic measures) were noted, neither in nine measured cytokines.
No clinically significant safety findings were observed in the 6 month follow-up period. - Single-dose administration of ALN-PCS yielded a rapid and dose-dependent reduction in plasma PCSK9 protein. The duration of the PCKS9-lowering effect was dose-dependent.
At the highest dose of 0.400 mg/kg, PCSK9 dropped with 70% from baseline, as compared to placebo treatment, on day 3 post-dose (P<0.0001). PCSK9 levels increased significantly in the placebo group, attributed to the pre-medication given to all participants. This effect was overcome with ALN-PCS treatment. - Reduction of plasma levels of PCSK9 protein was independent of baseline levels, which may vary by a factor of over 100 across a population.
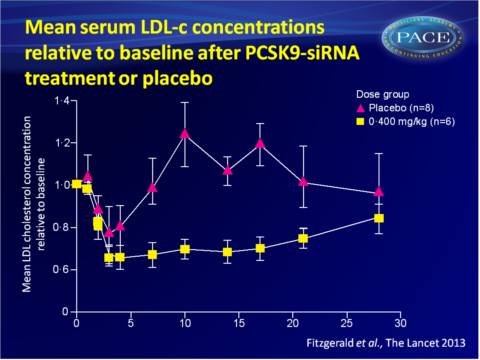
- Single-dose administration of ALN-PCS also yielded a rapid and dose-dependent drop in LDL-c levels, again with a dose-dependent duration. A mean 40% reduction was obtained with the highest dose.
Download Fitzgerald lancet 2013 PACE.pptx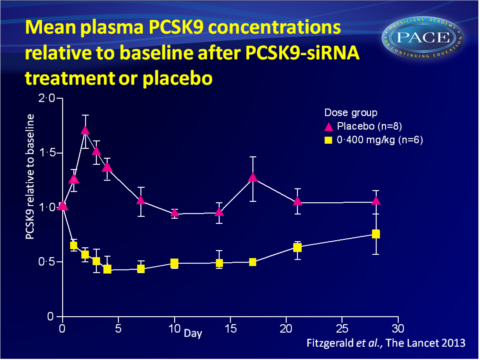
Conclusion
This first-in-man study showed that ALN-PCS was well-tolerated; the few mild to moderate adverse that were seen related to the treatment, were observed in both the ALN-PCS and placebo groups. No other clinically relevant changes in liver function or inflammatory markers were noted.Despite the fact that this study was underpowered to do so, significant decreases were observed in PCSK9 and LDL-c plasma levels in the higher dose group. The effects were rapid and durable.
References
1. Foley KA, Simpson RJ Jr, Crouse JR 3rd, et al. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol 2003; 92: 79–81.
2. Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet 2005; 366: 1267–78.
3 Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy of cholesterol-lowering therapy in 18 686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371: 117–25.
4 Genest J, McPherson R, Frohlich J, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult—2009 recommendations. Can J Cardiol 2009; 25: 567–79.
4. Foody JM, Sajjan SG, Hu XH, et al. Loss of early gains in low density lipoprotein cholesterol goal attainment among high-risk patients. J Clin Lipidol 2010; 4: 126–32.
5. Horton JD, Cohen JC, Hobbs HH. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem Sci 2007; 32: 71–77.
6. Poirier S, Mayer G, Poupon V, et al. Dissection of the endogenous cellular pathways of PCSK9-induced low density lipoprotein receptor degradation: evidence for an intracellular route. J Biol Chem 2009; 284: 28856–64.
7. Rashid S, Curtis DE, Garuti R, et al. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci USA 2005; 102: 5374–79.
8. Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003; 34: 154–56.
9. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354: 1264–72.
10. Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009; 50 (suppl): S172–77.
11. Careskey HE, Davis RA, Alborn WEet al. Atorvastatin increases human serum levels of proprotein convertase
subtilisin/kexin type 9. J Lipid Res 2008; 49: 394–98.
12. Davignon J, Dubuc G. Statins and ezetimibe modulate plasma proprotein convertase subtilisin kexin-9 (PCSK9) levels. Trans Am Clin Climatol Assoc 2009; 120: 163–73.
13. Costet P, Hoff mann MM, Cariou B, et al. Plasma PCSK9 is increased by fenofi brate and atorvastatin in a non-additive fashion in diabetic patients. Atherosclerosis 2010; 212: 246–51.
14. Welder G, Zineh I, Pacanowski MA, et al. High-dose atorvastatin causes a rapid sustained increase in human
serum PCSK9 and disrupts its correlation with LDL cholesterol. J Lipid Res 2010; 51: 2714–21.
15. Meister G, Tuschl T. Mechanisms of gene silencing by doublestranded RNA. Nature 2004; 431: 343–49.
16. Vaishnaw AK, Gollob J, Gamba-Vitalo C, et al. A status report on RNAi therapeutics. Silence 2010; 1: 14.
17. Frank-Kamenetsky M, Grefhorst A, Anderson NN, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci USA 2008; 105: 11915–20.
18. Jayaraman M, Ansell SM, Mui BL, et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem Int Ed Engl 2012; 51: 8529–33.
