Renal denervation persistently lowers blood pressure at least 36 months
36 months follow-up of RDN-treated patients and 30 months follow-up of RDN-treated patients who were initially in the control group of SYMPLICITY HTN-2, shows durable BP lowering effect.
Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trialLiterature - Esler MD et al., Eur Heart J. 2014 - Eur Heart J. 2014 Jul 7;35(26):1752-9
Esler MD, Böhm M, Sievert H, et al.
Eur Heart J. 2014 Jul 7;35(26):1752-9Background
Patients with treatment-resistant hypertension (blood pressure (BP) > 140/90 mmHg despite >3 antihypertensive medications, including a diuretic), often have other cardiovascular (CV) risk factors, thus putting them at further risk for end-organ damage and CV morbidities [1]. Estimates of truly treatment-resistant hypertension range from 5 to 16% [2-6].Renal sympathetic outflow is often overactive in patients with essential hypertension, and it was initially shown in experimental animal models that surgical sectioning of the renal nerves lowered BP [7,8].
Percutaneous catheter-based renal denervation (RDN) involves the application of low-power (~8W) radiofrequency energy to the main renal arteries in a helical pattern of ablations [9,10]. The SYMPLICITY HTN-1 proof-of-principle trial showed the feasibility of the procedure, and patients with severe treatment resistant hypertension had sustained BP reduction for at least 3 years [11-13].
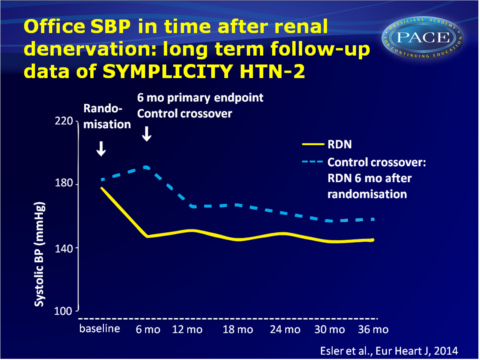
The SYMPLICITY HTN-2 was a randomised clinical that compared the safety and effectiveness of RND plus medical management to medical management alone (control group) in patients with severe treatment-resistant hypertension [14,15]. A significant decrease from baseline BP was seen 6 months after patients were randomised to RDN (-32/-12 mmHg, P<0.0001), while no significant change in BP was seen in the control group. The SYMPLICITY HTN-2 trial allowed control subjects to crossover to get RDN after the initial 6-month primary endpoint evaluation. This publication reports the 36-month follow-up of the initial-RDN group (n=40), and 30-month follow-up of the crossover subjects who received RDN 6 months later (n=30).
Main results
- Baseline SBP was 178+18 mmHg in the RDN group and 191+20 mmHg in the crossover group.
- SBP and DBP measurements were significantly lower than the pre-procedure measurement at all time points (6, 12, 24, 30 and 36 months). Reduction in BP did not diminish during follow-up. After 30 months, change in SBP was -34 mmHg (95%CI: -40 to -27, P<0.01) and change in DBP was -13 mmHg (95%CI: -16 to -10, P<0.01). Reduction of BP was similar for the original RDN and the crossover subjects.
- Response rate of achieving an SBP reduction of > 20 mmHg was similar for original RDN subjects (72%) and crossover subjects (70%).
- Heart rate was slowed following RDN, and the decrease persisted until 36 months (mean decrease 4 bpm (95%CI: -8 to -0.1, P=0.04).
- At 30 months, the mean number of antihypertensive medications was lowered from 5.1+1.4 at baseline to 4.8+1.5 (P=0.06) medications.
- Complications associated with the procedure included one haematoma, and one renal artery dissection before energy delivery, which could be treated successfully. Between 12 and 26 months after the procedure, five hypertensive events requiring hospitalisation and one case of mild transient acute renal failure were reported. Three deaths occurred during follow-up, which were considered unrelated to the device or therapy.
Download Esler EHJ 2014 PACE.pptx
Conclusion
This report adds long-term data on the safety and efficacy of RDN, by confirming the durability of the antihypertensive effects of RDN as shown in the earlier SYMPLICITY HTN-1 trial, in patients with severe treatment-resistant hypertension. The long-term effect was obtained without increasing medication, and without serious long-term safety concerns.These data were in contrast with the shorter-term SYMPLICITY HTN-3 trial, in which no significant difference was seen between RDN and a sham procedure. The better trained operators in the centres participating in SYMPLICTY HTN-2 may in part explain this difference.
Find this article on Pubmed
References
1. Calhoun DA, Jones D, Textor S, et al., American Heart Association Professional Education C. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008;117:e510–e526.
2. Sarafidis PA, Bakris GL. Resistant hypertension: an overview of evaluation and treatment. J Am Coll Cardiol 2008;52:1749–1757.
3. Wojciechowski D, Papademetriou V, Faselis C, Fletcher R. Evaluation and treatment of resistant or difficult-to-control hypertension. J Clin Hypertens (Greenwich) 2008;10: 837–843.
4. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 2012;125:1635–1642.
5. Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011;57:1076–1080.
6. de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension 2011;57: 898–902.
7. DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol 2010;298:R245–R253.
8. Foss JD, Fink GD, Osborn JW. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension 2013;61:806–811.
9. Atherton DS, Deep NL, Mendelsohn FO. Micro-anatomy of the renal sympathetic nervous system: a human postmortem histologic study. Clin Anat 2012;25:628–633.
10. Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol 2011; 1:731–767.
11. Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009;373:1275–1281.
12. Symplicity HTNI. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917.
13. Krum H, Schlaich MP, Sobotka PA, et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet 2014;383: 622–629.
14. Esler MD, Krum H, Sobotka PA, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010;376:1903–1909.
15. Esler MD, Krum H, Schlaich M, et al. Symplicity HTNI. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation 2012;126:2976–2982.
