Review on Cardiorenal syndromes
Cardiorenal syndromes (CRS) have been subclassified as five defined entities which represent clinical circumstances in which both the heart and the kidney are involved in a bidirectional injury and dysfunction via a final common pathway of cell-to-cell death and accelerated apoptosis mediated by oxidative stress.
Cardiorenal syndromesNews - Apr. 25, 2011
Peter A McCullough, Aftab Ahmad
Cited from World J Cardiol 2011; 3(1): 1-9
For full article visit http://www.wjgnet.com
Cardiorenal syndromes (CRS) have been subclassified as five defined entities which represent clinical circumstances in which both the heart and the kidney are involved in a bidirectional injury and dysfunction via a final common pathway of cell-to-cell death and accelerated apoptosis mediated by oxidative stress. Types 1 and 2 involve acute and chronic cardiovascular disease (CVD) scenarios leading to acute kidney injury or accelerated chronic kidney disease. Types 2 and 3 describe acute and chronic kidney disease leading primarily to heart failure, although it is possible that acute coronary syndromes, stroke, and arrhythmias could be CVD outcomes in these forms of CRS. Finally, CRS type 5 describes a simultaneous insult to both heart and kidneys, such as sepsis, where both organs are injured simultaneously. Both blood and urine biomarkers are reviewed in this paper and offer a considerable opportunity to enhance the understanding of the pathophysiology and known epidemiology of these recently defined syndromes.
INTRODUCTION
Both cardiac and renal diseases commonly present in thesame patient and have been associated with increased costs of care, complications, and mortality[1,2]. Cardiorenal syndromes (CRS), describing the dynamic inter-relationship between heart and kidney malfunction have been defined in a recent consensus process by the Acute Dialysis Quality Initiative (ADQI)[3]. This has generated five distinct syndromes upon which the epidemiology of CRS can be described. This paper will review this new classification and give concrete examples of each CRS, and discuss the available data on incidence and risk predictors.Finally, a succinct review of promising biomarkers will be presented that are very likely to change the described CRS epidemiological literature as we know it, based largely upon the measurement of a single blood biomarkerserum creatinine.
Classification of Cardiorenal Syndromes
The term cardiorenal syndromes suggests the presence of multiple syndromes with subtypes denoted by dysfunction of the principal organ (cardiac or renal or both) as well as the relative acuity of the condition. Both organs must have or develop pathological abnormalities to fulfill the criteria for definition. The umbrella term “cardiorenal syndromes” was defined as “Disorders of the heart and kidneys whereby acute or chronic dysfunction in one organ may induce acute or chronic dysfunction of the other”.
Five subcategories of CRS are given for each syndrome.
CATEGORIES OF SYNDROMES
The broad and most important concepts of CRS include the following:
- bidirectional organ injury or malfunction
- an inciting event for acute CRS
- a precipitous decline in function for acute or chronic CRS.
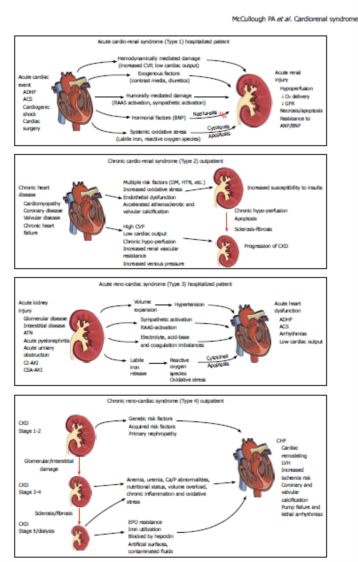
Acute cardiorenal syndrome
Acute cardiorenal syndrome (CRS Type 1): acute decompensation of cardiac function leading to acute renal failure. This is a syndrome of worsening renal function that frequently complicates acute decompensated heart failure (ADHF) and acute coronary syndrome (ACS). Seven observational studies have reported on the frequency and outcomes of CRS Type 1 in the setting of ADHF and five in ACS[4]. Depending on the population, 27%-40% of patients hospitalized for ADHF develop acute kidney injury (AKI) as defined by an increase in serum creatinine of ≥ 0.3 mg/dL[12,13]. Risk predictors for this complication include reduced baseline renal function, diabetes, and prior HF[14]. These patients experience more complicated hospital courses, longer inpatient stays, and higher mortality.In the Prospective Outcomes Study in Heart Failure (POSH) study, only in those with ADHF and a hospital course complicated by circulatory shock, hypotension, cardiac arrest, sepsis or ACS, a rise in serum creatinine did confer a higher 6-mo mortality[15]. Conversely, those with an increase in serum creatinine of ≥ 0.3 mg/dL but no other complications did not have higher mortality in the hospital, at 30 or 180 d. Thus, much of CRS Type 1 mortality is confounded by a complicated course and AKI. Importantly, it has been noted that CRS Type 1 in ADHF rarely occurs in the prehospital phase, and is observed after hospitalization, implying that some factor associated with hospitalization, namely diuresis, precipitates CRS. The use of loop diuretics, probably by further activation of the renin-angiotensin system and possibly worsening intra-renal hemodynamics, have been identified as one of the modifiable in-hospital determinants of CRS Type
1[16]. Testani et al[17] have recently shown in the Evaluation Study of Congestive Heart Failure and Pulmonary Artery
Catheterization Effectiveness (ESCAPE) trial that the use of higher doses of loop diuretics, causing hemoconcentration,
resulted in a 5-fold increased rate of worsening renal function. However, in this prospective trial of hemodynamic monitoring, aggressive diuresis was associated with a 69% reduction in mortality at 180 d. Several studies have
now linked the presence of an elevated central venous pressure and renal venous congestion to the development of CRS Type 1, thus, the relative balance of venous and arterial tone and congestion of the kidney appear to be important in the drop in renal filtration that occurs during hospitalized treatment of ADHF[18].
The other major clinical scenario where CRS Type 1 develops is in the setting of urgent or elective coronaryrevascularization for acute or chronic coronary disease. Acute contrast-induced and cardiopulmonary bypass surgery associated AKI occur in 15% and 30% of patients, respectively[19,20]. Importantly, iodinated contrast which causes renal vasoconstriction and direct cellular toxicity to renal tubular cells is an important pre-existing factor in the few days before cardiac surgery. Cardiac surgery exposes the kidneys to hypothermic, pulseless reduced perfusion for 30-90 min, and thus represents a superimposed ischemic injury in the setting of a pro-inflammatory state[21]. It is possible that the extracorporeal circuit used in cardiopulmonary bypass surgery activates systemic factors that further induce AKI; however, attempts to limit this exposure have not resulted in significantly reduced rates of AKI[22]. Thus, these two scenarios are tightly linked, since almost every cardiac surgery patient operated upon in the urgent setting undergoes coronary angiography in the hours to days before surgery[23]. As with ADHF, CRS Type 1 in acute and chronic coronary disease has a confounded relationship with outcomes. In those with complications, CRS Type 1 appears to be independently associated with a 3 to 4-fold increase in mortality despite the availability of dialysis in the hospital[24,25]. In all forms of CRS Type 1, there is a risk of advancing to higher stages of CKD and ultimately the need for chronic renal replacement strategies[26]. The incremental and cumulative risk of these renal outcomes according to the clinical scenarios described
above for an individual patient are unknown. Thus the important points concerning the epidemiology of CRS Type 1 are:
- the mortality risk appears to be confounded by other non-renal complications occurring during the hospitalization
- intravascular iodinated contrast alone, and in cases where cardiac surgery follows coronary angiography,
direct cellular toxicity from the contrast itself results in an observed rise in serum creatinine predominately in those
with baseline reductions in renal filtration with additional risk factors, including diabetes, heart failure, older age, and
larger contrast volumes - in the setting of ADHF, superimposed use of iodinated contrast or other cardiac procedures is associated with longer lengths of stay and higher mortality which is possibly in part, attributable to CRS Type 1[27-29].
Chronic cardiorenal syndrome
Chronic cardiorenal syndrome (CRS Type 2): chronic abnormalities in myocardial function leading to worsened chronic kidney disease (CKD). This subtype implies that chronic CVD can contribute to the development of CKD.
Six observation studies have reported on CRS Type 2, with a minority of reports reporting on CVD contributing to an excess risk of CKD[4]. It is recognized that the risk factors for atherosclerosis, namely diabetes, hypertension, and smoking are independently associated with the development of CKD[30]. In addition, chronic abnormalities in systolic and diastolic myocardial performance can lead to alterations in neurohormonal activation, renal hemodynamics, and a variety of adverse cellular processes leading to apoptosis and renal fibrosis[31]. Approximately 30% of those with chronic cardiovascular disease (CVD) meet a definition of CKD, and multiple studies have demonstrated the independent contribution of CVD to the worsening of CKD[32]. An important component of CRS Type 2 epidemiology is that CKD appears to accelerate the
course of atherosclerosis and result in premature CVD events including myocardial infarction and stroke[33,34].
Importantly, CKD and its metabolic milieu work to cause advanced calcific atherosclerosis through CKD mineral and bone disorder characterized by phosphate retention, relative vitamin D and calcium availability, and secondary yperparathyroidism[35]. Of these factors, phosphate retention appears to be the critical pathophysiological component
stimulating the conversion of vascular smooth muscle cells to osteoblastic-like cells which, via the Pit-1 receptor, are stimulated to produce extracellular calcium hydroxyapatite crystals in the vascular smooth muscle layer of arteries[36,37]. Thus, patients as a part of CRS type 2, more commonly have vascular calcification, less vascular compliance, and a higher degree of chronic organ injury related to blood pressure elevation and shear stress[38]. Despite these mechanisms specific to CRS, CRS Type 2 remains heavily confounded by the “common soil” of atherosclerosis and CKD. The cardiometabolic syndrome and neurohormonal activation affect both organ systems; thus, it is difficult to tease out the temporal sequence of pathophysiological events for most individuals which are occurring over the period of decades[39].
Studies have shown that 45.0%-63.6% of patients with chronic HF have evidence of CKD defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2[40]. Multiple studies have demonstrated that CKD is closely linked to more frequent hospitalizations and complications from pump failure and arrhythmias[41,42]. In addition, patients with CKD and end-stage renal disease have higher defibrillation thresholds and may not have the protective benefit of implantable cardio defibrillators as those with normal renal function[43]. Increased degrees of left ventricular hypertrophy and cardiac fibrosis are believed to be the biologic basis for these electrophysiological findings[44].
Acute renocardiac syndrome
Acute renocardiac syndrome (CRS Type 3): acute worsening of renal function leading to cardiac events. The most common scenario for CRS Type 3 is the development of AKI that results in volume overload, sodium retention, neurohormonal activation, and the development of clinical HF with the cardinal features of pulmonary congestion and peripheral edema. Volume overload alone has been shown to induce cardiac failure and reflect CRS Type 3 most clearly in the pediatric population[45]. However, in adults, when acute on chronic disease is a common occurrence, it is difficult to identify clear cases where AKI lead to cardiac decompensation. It is also possible that CRS Type 3 could precipitate in an acute coronary syndrome, stroke, or other acute cardiac event. Thus the epidemiology of this CRS subtype is not well defined for individual CVD events such as ACS, stroke, cardiac rehospitalization, arrhythmias, pump failure, and cardiac death[4].
Chronic renocardiac syndrome
Chronic renocardiac syndrome (CRS Type 4): chronic renal disease leading to the progression of cardiovascular disease. Over the past several decades there has been recognition of a graded and independent association between the severity of CKD and incidence as well as prevalence of CVD[2]. In a meta-analysis of 39 studies (1 371 990 participants), there was a clear relationship between the degree of renal dysfunction and the risk for all-cause mortality[46]. The unadjusted relative risk of mortality in participants with reduced kidney function was in excess of the reference group in 93% of cohorts.
Fourteen of the 39 studies described the risk of mortality from reduced kidney function, after adjustment for other established risk factors. Although adjusted relative hazard ratios were on average 17% lower than unadjusted relative
risks, they remained significantly greater than unity in 71% of cohorts. The overall mortality was influenced greatly by
excess cardiovascular deaths, which constituted over 50% of cases. Thirteen studies have been identified as specifically
reporting on CRS Type 4, most of which were in populations with end-stage renal disease[4]. It should also be recognized, that CKD contributes to CVD outcomes in CRS Type 4 by complicating pharmacological and interventional treatment[47,48]. For example, azotemia and hyperkalemia restrict the use of drugs that antagonize the renin-angiotensin system, thus fewer patients with CKD enjoy the cardiovascular benefits of angiotensin converting enzyme inhibitors, angiotensin Ⅱ receptor antagonists, and aldosterone receptor blockers[49,50]. It has been shown that CKD also worsens the presentation, severity, response to treatment, and cardiorenal outcomes in acute and chronic hypertension[51,52]. In addition, the perceived risks of AKI lead patients with CKD towards conservative management strategies which have been associated with poor outcomes in the setting of both acute and chronic coronary artery disease[53]. Finally, a recent study
of silent brain injury (asymptomatic cerebral infarctions by magnetic resonance imaging) has been associated with
a rapid decline in renal function in approximately 30% of patients[54]. This suggests the possibility that cerebrovascular
disease could in some way contribute to more rapid progression of CKD.
Secondary cardiorenal syndrome
Secondary cardiorenal syndrome (CRS Type 5): systemic illness leading to simultaneous heart and renal failure. It is recognized that a systemic insult, particularly in a younger patient with no prior heart or kidney disease, can lead to simultaneous organ dysfunction. This is almost always in the setting of critical illness such as sepsis, multiple trauma, or burns. There are limited data on the incidence and determinants of CRS Type 5, in part because of confounders such as hypotension, respiratory failure, liver failure, and other organ injury beyond the cardiac and renal systems. This results in a difficult human model for investigation. Sepsis as a precipitator of CRS Type 5 is common and its incidence is increasing, with a mortality estimated at 20%-60%[55,56]. Approximately 11%-64% of septic patients develop AKI that is associated with a higher morbidity and mortality[57]. Abnormalities in cardiac function are also common in sepsis including wall motion abnormalities and transient reductions in left ventricular ejection fraction[58]. Observational data have found approximately 30%-80% of individuals with sepsis have measurable blood troponin I or T that are above the 99th detection limits[59]. These elevated cardiac biomarkers have been associated with reduced left ventricular function and higher mortality even in patients without known coronary disease[60-62]. Importantly, volume overload as a result of aggressive fluid resuscitation appears to be a significant determinant of CRS Type 5. Among 3147 patients enrolled in the Sepsis Occurrence in Acutely Ill Patients (SOAP), there was a 36% incidence of AKI, and volume overload was the strongest predictor of mortality[60]. Iatrogenic volume overload appears to play an important additional role, possibly along the lines described for CRS Type 1 and passive venous congestion of the kidney, in the pathogenesis of AKI. At the same time, volume overload increases left ventricular wall tension and likely contributes to cardiac decompensation in those predisposed to both systolic and diastolic HF[61]. In summary for CRS Type 5, both AKI and markers of cardiac injury followed by volume overload are common in sepsis, with each being associated with increased mortality. However, there is a current lack of integral information on the incidence of bidirectional organ failure and its pathophysiological correlates in a variety of acute care settings.