SGLT2 inhibition lowers CV risk in type 2 diabetic patients with and without CVD
Type 2 diabetic patients treated with SGLT2i had a lower risk of death and HF when compared with other glucose-lowering drugs, regardless of the presence of CVD.
SGLT-2 Inhibitors and Cardiovascular Risk: An Analysis of CVD-REALLiterature - Cavender MA, Norhammar A, Birkeland KI, et al. - J Am Coll Cardiol 2018;71:2497–506
Introduction and Methods
Sodium-glucose co-transporter-2 inhibitors (SGLT2i) reduce CV risk in high risk type 2 diabetes mellitus (T2DM) patients [1,2]. The CVD-REAL (Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors) real world study showed that SGLT2i treatment is associated with a lower risk of death and heart failure (HF) compared with other glucose-lowering drugs (GLDs) [3], however it is not clear whether this association is independent on the presence of CVD.
The associations among SGLT2i, death, and HF were assessed according to the presence or absence of CVD at the time of initiation of glucose-lowering therapy in this analysis of the CVD REAL study. For this purpose, data from medical records, medical claims, electronic health and death records, and national registers were used, and adult patients with T2DM were identified.
Patients with type 1 or gestational diabetes were excluded from the analysis. Eligible patients were newly initiated on one of the SGLT2i canagliflozin, dapagliflozin, or empagliflozin, or other GLDs, and were matched 1:1 based on propensity scores.
Intention-to-treat analyses were done, starting at the beginning of glucose-lowering therapy until the first CV event or until the end of follow-up, and an additional on-treatment analysis served as a sensitivity analysis. Patients were stratified based on the presence or absence of established CVD, defined as a history of acute myocardial infarction, unstable angina, stroke, HF, transient ischemic attack, coronary revascularization, or occlusive peripheral artery disease. The primary endpoints included time to death, HF, and the composite endpoint of HF or death.
Main results
- A total of 306,156 patients were included in the analysis. The mean follow-up time ranged from 313-387 days in patients treated with SGLT2i and 299-383 days in patients treated with other GLDs.
- In the SGLT2i group, 53.2% of patients received canagliflozin, 41.4% received dapagliflozin, and 5.4% received empagliflozin.
- In the GLD group 33.7% received insulin, 17.3% received dipeptidyl peptidase-4 inhibitors, 17.1% received sulfonylureas, 13.8% received glucagon-like peptide-1 receptor, and 11.4% received metformin.
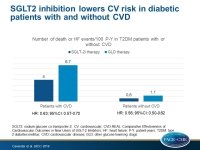
- Compared with other GLDs, the use of SGLT2i was associated with lower risk of death in patients with CVD (1.8 vs. 3.6 events per 100 patient-years; HR: 0.56; 95%CI: 0.44-0.70), as well as in patients without CVD (0.5 vs. 0.9 events per 100 patient-years; HR: 0.56; 95%CI: 0.50-0.63).
- Compared with other GLDs, SGLT2i therapy was associated with a lower risk of HF in both subgroups (with CVD: 2.3 vs. 3.2 events per 100 patient-years; HR: 0.72; 95%CI: 0.63-0.82; without CVD: 0.1 vs. 0.2 events per 100 patient-years; HR: 0.61; 95%CI: 0.48-0.78).
- Compared with other GLDs, patients on SGLT2i therapy had a lower risk of the combined endpoint with CVD (4.0 vs. 6.7 events per 100 patient years; HR: 0.63; 95%CI: 0.57-0.70), and without CVD (0.6 vs. 1.1 events per 100 patient years; HR: 0.56; 95%CI: 0.50-0.62).
- The on-treatment analysis resulted into similar findings.
Conclusion
T2DM patients included in the real world CVD-REAL study had a lower risk of death and HF when treated with SGLT2i compared with other GLDs, regardless of the presence of CVD. These findings suggest that the CV benefits of SGLT2i therapy may apply for the whole class, and both for patients in primary and secondary prevention.
Editorial comment
In their editorial article [4], Farkouh and Verma point at the consistency of the CVD-REAL results with those of other SGLT2i studies, like the EMPA-REG and the CANVAS programs, and they discuss the cardio-protective mechanisms of SGLT2 inhibition, including diuresis, natriuresis, glycosuria, pre- and afterload reductions, ketone metabolism, and direct myocardial effects, like improved diastolic function and left ventricular mass. The authors conclude: ‘We congratulate the authors for this important subanalysis, which continues to remind us of the importance of heart failure prevention in individuals with diabetes, while ushering hope that SGLT2i may be a preferred agent of choice in the prevention of one of the deadliest complications of diabetes……. Further insights from the CVD-REAL 2 study are also eagerly awaited. Whether SGLT2i will be useful agents in the treatment (vs. prevention) of heart failure with and without diabetes remains an important question for which ongoing studies are currently underway.’
References
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28.
2. Neal B, Perkovic V, Mahaffey KW, et al. Optimizing the analysis strategy for the CANVAS program: a prespecified plan for the integrated analyses of the CANVAS and CANVAS-R trials. Diabetes Obes Metab 2017;19:926–35.
3. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVDREAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation 2017;136:249–59.
4. Farkouh M, and Verma S. HF Prevention With SGLT-2 Inhibition. JACC 2018;71(22):2507-10.