SGLT2 inhibitor lowers risk of microvascular events and renal impairment in high risk T2DM patients
EMPA-REG OUTCOME trial shows that empagliflozin reduces microvascular outcomes and progression of kidney disease in T2DM patients at high CV risk.
Empagliflozin and Progression of Kidney Disease in Type 2 DiabetesLiterature - Wanner C et al., NEJM 2016
Wanner C, Inzucchi SE, Lachin JM et al., for the EMPA-REG OUTCOME Investigators
NEJM June 14, 2016DOI: 10.1056/NEJMoa1515920
Background
Approximately 35% of patients with type 2 diabetes (T2DM) develop kidney disease [1], which is associated with increased mortality [2]. Surrogate markers of renal complications have been shown to be reduced in response to intensive glucose-lowering strategies in T2DM patients, but evidence for improvement in advanced renal complications is limited [3-6].Empagliflozin, a selective sodium-glucose cotransporter 2 (SGLT2) inhibitor, reduces renal reabsorption of glucose, thereby increasing urinary glucose excretion. This reduces hyperglycemia in patients with T2DM. Treatment with empagliflozin has been associated with lower glycated haemoglobin levels in T2DM, including in patients with stage 2 or 3a chronic kidney disease [7-14]. In patients with type 1 diabetes, empagliflozin has been shown to reduce intraglomerular pressure and improve hyperfiltration [15,16]. While these effects may translate to improved renal outcomes, concern has also been expressed that SGLT2-inhibitors may be associated with long-term adverse renal effects.
The EMPA-REG OUTCOME trial [17] assessed CV outcomes, and found a significantly lower risk of 3-point MACE in those treated with empagliflozin as compared with placebo. The difference was driven by a lower rate of CV death [17].
This paper reports on a prespecified secondary objective of the trial, to examine the effects of empagliflozin on microvascular outcomes and progression of kidney disease in patients with T2DM at high CV risk. Included patients had eGFR of at least 30 ml/min/1.73m2 of body surface area according to the MDRD formula. This report focusses on renal microvascular outcomes, among which incident or worsening nephropathy, defined as progression to macroalbuminuria (urinary albumin to-creatinine ratio, >300 mg of albumin per gram of creatinine); a doubling of the serum creatinine level, accompanied by an eGFR of ≤45 ml/minute/per 1.73 m2; the initiation of renal-replacement therapy; or death from renal disease.
Main results
- The composite microvascular outcome (including retinal and renal outcomes) occurred in 577 of 4132 (14.0%) in the empagliflozin group and in 424 of 2068 patients (20.5%) in the placebo group, implying a relative risk reduction of 38% (HR: 0.62, 95%CI: 0.54-0.70, P<0.001). This effect was driven by the renal component.
- Incident or worsening nephropathy occurred in 525 of 4124 patients (12.7%) in the empagliflozin group and in 388 of 2061 patients (18.8%) in the placebo group, yielding a significant relative risk reduction of 39% (HR: 0.61, 95%CI: 0.53-0.70, P<0.001).
- Progression to macroalbuminuria occurred in 459 of 4091 patients on empagliflozin and in 330 of 2033 (16.2%) on placebo, meaning a relative risk reduction of 38% (HR: 0.62, 95%CI: 0.54-0.72, P<0.001).
- Doubling of serum creatinine level occurred less often with empagliflozin than in the placebo group (1.5% vs. 2.6%, HR: 0.56, 95%CI: 0.39-0.79, 0.001), as did initiation of renal-replacement therapy (0.3% vs. 0.6%, HR: 0.45, 95%CI: 0.21-0.97, P=0.04).
- A post hoc sensitivity analysis and a subgroup analysis in patients with prevalent kidney disease at baseline confirmed the results for the composite renal outcomes.
- Similar patterns of renal function (eGFR) over time were seen in patients with eGFR >60 ml/min/1.73m2 and in patients with eGFR <59 ml/min/1.73m2 at baseline.
- The empagliflozin group showed a short-term decrease in eGFR from baseline to week 4, which was reversed after cessation of the study drug. At the follow-up visit, adjusted mean difference from placebo in the change in eGFR from baseline was 4.7 ml/min/1.73m2 (95%CI: 4.0-5.5, P<0.001 for comparisons of both doses with placebo).
- Adverse events, serious adverse events or adverse events leading to study-drug continuations were similar in the treatment arms, and in the eGFR groups. Genital infections were reported more often by patients in the empagliflozin group than in the placebo group.
Download Wanner NEJM 2016_EMPA-REG OUTCOME renal_PACE.pptx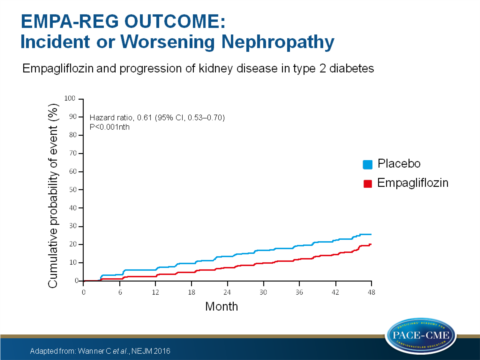
Conclusion
Patients with T2DM at high risk for CV events who were treated with empagliflozin in addition to standard care, had a significantly lower risk of microvascular outcome events than did patients receiving placebo. The difference could be attributed to a lower risk of progression of kidney disease (incident or worsening nephropathy). Patients receiving empagliflozin also less often progressed to macroalbuminuria or clinically relevant outcomes.Find this article online at NEJM
References
1. de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011; 305: 2532-9.
2. Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013; 24: 302-8.
3. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837-53.
4. Coca SG, Ismail-Beigi F, Haq N, Krumholz HM, Parikh CR. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med 2012; 172: 761-9.
5. The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560-72.
6. Perkovic V, Heerspink HL, Chalmers J, et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int 2013; 83: 517-23.
7. Zoungas S, Chalmers J, Neal B, et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med 2014; 371: 1392-406.
8. Häring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2013; 36: 3396-404.
9. Häring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2014; 37: 1650-9.
10. Kovacs CS, Seshiah V, Swallow R, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab 2014; 16: 147-58.
11. Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, doubleblind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2013; 1: 208-19.
12. Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care 2014; 37: 1815-23.
13. Rosenstock J, Jelaska A, Zeller C, Kim G, Broedl UC, Woerle HJ. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 2015; 17: 936-48.
14. Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 2015; 38: 420-8.
15. Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2014; 2: 369-84.
16. Skrtić M, Yang GK, Perkins BA, et al. Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia 2014; 57: 2599-602.
17. Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014; 129: 587-97.
18. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117-28.
