Similar resources required for management of major bleeds on dabigatran as on warfarin, with better outcome on dabigatran
Patients with major bleedings on dabigatran were at higher risk than those with bleeds on warfarin, but the stay at intensive care is shorter with dabigatran treatment and 30day mortality tends to be lower.
Management and Outcomes of Major Bleeding during Treatment with Dabigatran or WarfarinLiterature - Majeed A,Hwang H-G,Connolly SJ - Circulation Sept. 30 2013
Majeed A , Hwang H-G, Connolly SJ et al.
Circulation Sept. 30 2013 online before print. Doi: CIRCULATIONAHA.113.002332
Background
Dabigatran etexilate has been approved in over 80 countries for stroke prevention in atrial fibrillation, based on the results of the RELY trial [1]. Major bleeding frequency at the highest dose (150 mg) was similar to warfarin and at the lower dose (110 mg) it was less frequent as compared to warfarin. A lower bleeding rate was seen with dabigatran 150 mg twice daily as compared to warfarin (INR: 2.0-3.0) in patients with venous thromboembolism (VTE) [2].Most vitamin K antagonists have a long half-life, but their anticoagulant effects can be reversed within 10-20 minutes by prothrombin complex concentrates (PCCs) and within 6-12 hours by vitamin K [3]. No antidote exists for dabigatran, but it has a half-life of 12-14 hours [4] and withholding the drug for 1-2 days is sufficient to restore haemostasis in most cases of mild to moderate bleeding. In more life-threatening cases, more rapid restoration of haemostasis might be achieved by haemodialysis to remove the drug [5-7], activated charcoal to prevent gastrointestinal absorption of recently ingested drug [8] and the administration of PCCs [9], activated PCCs [10] or recombinant activated factor VII (fFVIIa) [11] to enhance thrombin generation. Evidence on the efficacy of these approaches is, however, limited.
In the absence of an effective antidote many clinicians are concerned that dabigatran-treated patients who experience major bleedings cannot be adequately managed. This study examined the management of major bleeding and outcomes after bleeding in large phase III trials (RE-LY, RE-COVER, RE-COVER II, Re-MEDY and RE-SONATE) evaluating the efficacy and safety of long-term dabigatran, in comparison with warfarin. A total of 1121 major bleeding events (in 1034 patients) were analysed.
Main results
- Patients with major bleeding during treatment with dabigatran were significantly older (75.3 years) and had lower creatinin clearance (median 53 mL/min) than those who had major bleeding on warfarin treatment (71.8 years, 62 mL/min). Furthermore, a larger proportion of dabigatran-treated patients who experienced bleeding had concomitant aspirin (30.9%) or an NSAID (12.9%) than patients on warfarin (24.6% and 8.4% respectively).
- In 365 (33%) major bleeding events no blood products or other haemostatic agents were given. Red blood cell transfusions alone was more often given in dabigatran-treated patients, for 395 bleeding events (35%)(dabigatran 110 mg: 137 of 293 bleeds [47%], dabigatran 150 mg: 173 of 403 bleeds [43%], warfarin: 85 of 425 bleeds [20%]).
- A minority of patients received any haemostatic therapy, i.e. plasma, vitamin K, factor concentrates, cryoprecipitate or platelets. In RE-LY patients with major bleeding events, fresh frozen plasma or vitamin K was less often used in the dabigatran group than in the warfarin group. A similar picture was seen in the VTE studies. Use of cryoprecipitate, platelets, PCC, rFVIIa or other coagulation factors was overall low and not different between treatments.
- In RE-LY, duration of stay in intensive care units was shorter for patients on dabigatran (mean: 1.6 nights) than for patients on warfarin (2.7 nights, P=0.01).
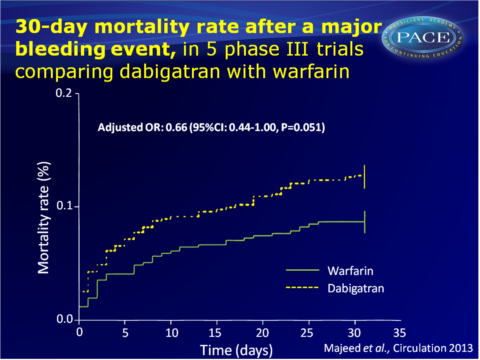
- Adjusted OR for 30-day mortality was 0.66 (95%CI: 0.44-1.00, P=0.051) for the combined dabigatran doses vs. warfarin. Dabigatran 110 mg alone gave OR: 0.65 (95%CI: 0.36-0.86, P=0.009) and 150 mg showed OR: 0.68 (95%CI: 0.42-1.08).
The RE-LY population showed similar, significant adjusted ORs for dabigatran, while patients with VTE showed no reduction of mortality upon dabigatran use, but the number of events was small. - In the RE-LY trial, no difference was seen between the treatment arms in discharge destination (home, long term facility or other hospital).
Download Majeed circ 2013 PACE.pptxor click to enlarge
Conclusion
This study shows that patients who experience major bleeding when on dabigatran treatment, are generally at higher risk as compared to patients with major bleeding events on warfarin. Some of these bleeds may therefore be preventable, by using a lower dose of dabigatran, as per some treatment guidelines, and by avoiding concomitant medication of aspirin an d NSAIDs.Most major bleeds were managed with supportive care only; the overall resources required to manage bleeding were not greater for dabigatran treatment than warfarin therapy. The outcome of major bleeding events on dabigatran treatment was better than on warfarin, since patients on dabigatran stayed shorter in intensive care units and a trend towards lower adjusted all-cause mortality at 30 days was seen.
Thus, dabigatran offers an alternative to warfarin with at least similar efficacy, similar or lower risk of major bleeding (specifically intracranial haemorrhage), that can be managed satisfactorily with simple measures, yielding a trend to lower mortality after such bleeding events.
References
1. Connolly SJ, Ezekowitz MD, Yusuf S, et al, the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
2. Schulman S, Kearon C, Kakkar AKet al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism.N Engl J Med. 2009;361:2342-2352.
3. Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e152S-184S.
4. Stangier J, Rathgen K, Stahle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64:292-303.
5. Stangier J, Rathgen K, Stahle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallelgroup, single-centre study. Clin Pharmacokinet. 2010;49:259-268.
6. Warkentin TE, Margetts P, Connolly S, et al. Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood. 2012;119:2172-2174.
7. Khadzhynov D, Wagner F, Formella S, et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost. 2013;109:596-605.
8. van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate--a novel, reversible, oral direct thrombin inhibitor: interpretation ofcoagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116-1127.
9. Zhou W, Schwarting S, Illanes S, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke. 2011;42:3594-3599.
10. Dager W, Roberts A. Reversing dabigatran with FEIBA in a patient with a transseptal perforation during cardiac ablation. Crit Care Med. 2011;39 (Suppl):243.
11. van Ryn J, Kink-Eiband M, Clemens A. The successful reversal of dabigatran-induced bleeding by coagulation factor concentrates in a rat tail bleeding model do not correlate with ex vivo markers of anticoagulation. Proceedings of American Society of Hematology Conference,San Diego. 2011:Abstract 2316..
Find this article online
