Statin treatment not only lowers cholesterol but increases body weight and risk of type 2 diabetes
Both HMGCR genetic variants and statin treatment are associated with higher bodyweight and higher risk of T2DM, suggesting that both are a consequence of HMGCR inhibition, the intended effect of statins.
HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials.Literature - Swerdlow DI et al., Lancet. 2014 - Lancet. 2014 Sep 24
Swerdlow DI, Preiss D, Kuchenbaecker KB et al.,
Lancet. 2014 Sep 24. doi: 10.1016/S0140-6736(14)61183-1. [Epub ahead of print]
Background
A dose-related increased risk of type 2 diabetes mellitus (T2DM) with statin therapy has been identified in a meta-analysis of randomised controlled trials, as compared to placebo or standard care [1,2], which has been confirmed in observational studies [3-5]. Despite T2DM being a cardiovascular (CV) risk factor, statin treatment still has a net benefit for the prevention of CV disease, including among patients with diabetes [6].The mechanism underlying the glucose-raising effects of statins is as yet unclear. This study used the Mendelian randomisation principle to investigate this mechanism, with common variants in the gene encoding a drug target as unconfounded, unbiased proxies for pharmacological action on that target. Single nucleotide polymorphisms (SNPs) in the HMG CoA reductase (HMGCR) gene were identified, after which their associations with several phenotypes related to HMGCR inhibition were analysed. Furthermore, a meta-analysis of the effect of statins on T2DM risk in randomised trials was updated and complemented with information on body weight.
Main results
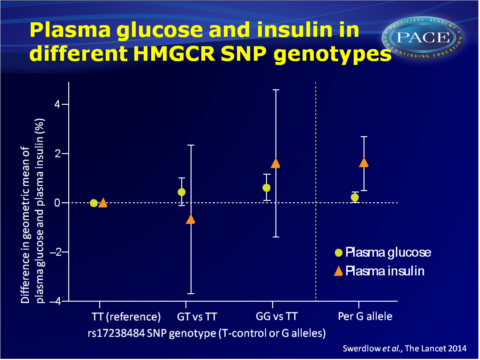
- The HMGCR rs17238484-G allele was associated with 1.62% (95%CI: 0.53–2.72, P=0.004) higher plasma insulin concentration (n=37.453, 12 studies) and with higher plasma glucose concentration (0.23%, 95%CI: 0.02–0.44; P=0.03; n=73.490, 23 studies).
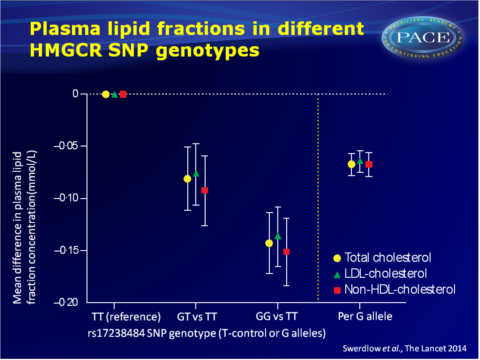
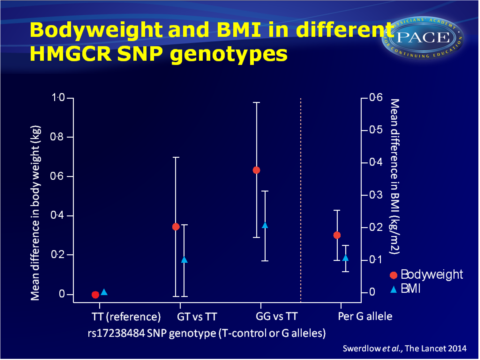
- The rs17238484-G allele was furthermore associated with higher bodyweight, high BMI, greater waist and hip circumference and waist:hip ratio, but not height.
- The rs12916 SNP showed directionally concordant associations.
- Both the rs17238484-G and the rs12916 allele appeared associated with an elevated risk of T2DM (OR per allele 1.02, 95%CI: 1.00-1.05, P=0.09 and OR: 1.06, 95%CI: 1.03-1.09, P=9.58x10-5).
- Analysis of combined data of 20 trials (mean follow-up of 4.2 years, range: 1.9-6.7) yielded an OR for new-onset T2DM with statin treatment of 1.12 (95%CI: 1.06-1.18), with little heterogeneity between trial-specific ORs.
- In a random effects meta-analysis of data of 15 trials (91393 individuals without T2DM at baseline) showed that statin-treated individuals were 0.24 kg (95%CI: 0.10-0.38) heavier at the end of the mean follow-up of 3.9 years (range 1.9-5.9) than controls, but with substantial heterogeneity between trials.
Download Swerdlow lancet 2014-PACE.pptx
Conclusion
This study shows that both HMGCR genetic variants in population studies and statin treatment in trials are associated with higher bodyweight and higher risk of T2DM, suggesting that these are a consequence of HMGCR inhibition, the intended effect of statins.The newly discovered association of statin treatment and increased bodyweight may partly explain the increased risk of T2DM in statin-treated patients, but it is unclear whether the relation between HMGCR inhibition and T2DM exclusively runs via changes in body composition. These results underscore the need for lifestyle interventions such as bodyweight optimisation, healthy diet and adequate physical activity, in order to attenuate the risk of type 2 diabetes.
Editorial comment [7]
“Swerdlow and colleagues provide a new angle to the debate about the adverse side-effects of statins.” (…) None of the previous “studies could establish whether or not the diabetogenic effect of statins operated through the same pathway as the lipid-lowering HMGCoA-reductase effect or an off-target effect. Swerdlow and colleagues answer this question—because the genetic variant lies near HMGCR, the diabetogenic effect of statins probably operates through the same mechanism as the lipid-lowering effect. (…) These results are important because they suggest that any attempts to make statins more specific and reduce off-target effects will not reduce the risk of the diabetogenic side-effect.”Find this article on Pubmed
References
1 Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010; 375: 735–42.
2 Preiss D, Seshasai SRK, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011; 305: 2556–64.
3 Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ 2013; 346: f2610.
4 Navarese EP, Buff on A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol 2013; 111: 1123–30.
5 Danaei G, Garcia Rodriguez LA, Fernandez Cantero O, Hernan MA. Statins and risk of diabetes: an analysis of electronic medical records to evaluate possible bias due to differential survival. Diabetes Care 2013; 36: 1236–40.
6 Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371: 117–25.
7 Frayling TM. Statins and type 2 diabetes: genetic studies on target. The Lancet, Early Online Publication, 24 September 2014 doi:10.1016/S0140-6736(14)61639-1
