Treatment effect of rivaroxaban vs. warfarin not affected by cTTR
A wide range of center-TTRs were seen in ROCKET-AF, but the relative efficacy of rivaroxaban over warfarin did not vary with cTTR, for stroke prevention in atrial fibrillation.
Relationship between time in therapeutic range and comparative treatment effect of rivaroxaban and warfarin: results from the ROCKET AF trialLiterature - Piccini JP, J Am Heart Assoc. 2014 - J Am Heart Assoc. 2014 Apr 22;3(2):e000521
Piccini JP, Hellkamp AS, Lokhnygina Y et al. ROCKET AF Investigators
J Am Heart Assoc. 2014 Apr 22;3(2):e000521
Background
International normalised ratio (INR) is a measure of the degree of anticoagulation when vitamin K antagonists (VKAs) are used. An INR of between 2.0 and 3.0 is generally recommended to limit both the risk of stroke and bleeding [1,2]. Time in therapeutic range (TTR) of INR is considered a measure of quality of VKA anticoagulation in patients with atrium fibrillation (AF).A direct relationship has been shown between TTR and lower rates of stroke and systemic embolism (SE) in AF patients. The relationship between TTR and bleeding risk appears more variable [3,4].
The ROCKET-AF trial directly compared warfarin treatment with the novel factor Xa inhibitor rivaroxaban in a blinded, randomised fashion in high-risk population [5].
The relationships among TTR, outcomes and treatment effect cannot be directly assessed at the individual level, since individual TTR is not meaningful in patients treated with rivaroxaban. This pre-specified analysis of the ROCKET-AF trial compares treatment effects of rivaroxaban vs. warfarin stratified by centre TTR (cTTR), thus still benefitting from randomisation. This type of analysis only allows indirect inference about the effect of individual TTR.
Main results
- Mean individual TTR in warfarin –treated patients was 55%. Mean cTTR was 59% and median cTTR was 61% (IQR: 51%-69%). Centres with higher cTTR had patients lower CHADS2 scores, and a lower prevalence of prior stroke or transient ischaemic attack.
- Mean cTTR varied across geographic regions, as did the associated HR for the primary efficacy endpoint. Lowest mean cTTR was seen in Asia/Pacific Islands (cTTR: 52%, HR: 0.67, 95%CI: 0.44-1.03) and Eastern Europe (cTTR: 52%, HR: 0.88, 95%CI: 0.67-1.19). Highest mean cTTR was seen in Western Europe (cTTR: 64%, HR: 0.87, 95%CI: 0.52-1.46) and North America (cTTR: 65, HR: 0.61, 95%CI: 0.35-1.06). No interaction was seen between region and treatment (P(interaction)=0.62).
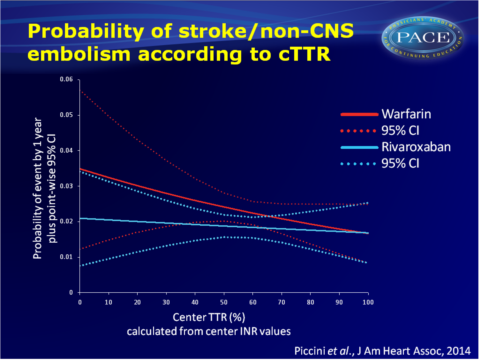
- Rates of stroke or non-central nervous system (CNS) embolism decreased with higher cTTR. The treatment effect of rivaroxaban vs. warfarin on the primary efficacy endpoint was consistent across cTTR quartiles (P(interaction)=0.71).
- Rates of the primary safety endpoint (major and non-major clinically relevant bleeding) increased with higher cTTR. There was a statistically significant interaction (P=0.001), with a lower hazard of bleeding with rivaroxaban in the lowest quartile of cTTR (HR: 0.80, 95%CI: 0.66-0.98) and a higher hazard of bleeding in the highest quartile (HR: 1.25, 95%CI: 1.10-1.41).
- Centers with a higher cTTR value gave a lower modelled risk of stroke or non-CNS embolism in both rivaroxaban- and warfarin-treated patients. The treatment effect of rivaroxaban vs. warfarin was relatively consistent across the range of cTTR values.
- There was no evidence that the benefit of rivaroxaban for prevention of intracranial bleeding is lost at any level of cTTR.
Download PICCINI JAHA 2014 pacepptx.pptx
Conclusion
TTR correlated negatively with risk of stroke and SE, and positively with risk of bleeding in the ROCKET-AF trial. It should be noted that centres with higher cTTR had patients with different comorbidity profiles, including lower-risk patients.Despite the fact that a wide range of TTR levels were observed across centres, no evidence was seen that the relative efficacy of rivaroxaban vs. warfarin varied with cTTR. Thus, while TTR is an important and validated quality measure for VKA management, it does not impact the estimate of the treatment effect of rivaroxaban vs. warfarin for the prevention of stroke and SE.
Find this article on Pubmed
References
1. Estes NA III, Halperin JL, Calkins Het al., ACC/AHA/Physician Consortium 2008 clinical performance measures for adults with nonvalvular atrial fibrillation or atrial flutter. Circulation. 2008;117:1101–1120.
2. Singer DE, Chang Y, Fang MC, Borowsky. Should patient characteristics influence target anticoagulation intensity for stroke prevention in nonvalvular atrial fibrillation? The ATRIA study. Circ
Cardiovasc Qual Outcomes. 2009;2:297–304.
3. Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–1026.
4. Samsa GP, Matchar DB. Relationship between test frequency and outcomes of anticoagulation: a literature review and commentary with implications for the design of randomized trials of patient self-management. J Thromb Thrombolysis. 2000;9:283–292.
5. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891.
