Twincretin: Superior glycemic control and weight loss compared to GLP-1RA monotherapy in T2DM
In a phase 2b randomized trial, LY3298176, a dual GIP and GLP-1 RA, led to a statistically significant and clinically meaningful dose-dependent improvement of glucose lowering and body weight reduction compared with dulaglutide.
Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trialLiterature - Frias JP, Nauck MA, Van J et al. - The Lancet 2018; published online ahead of print
Introduction and methods
Glucagon-like peptide-1 receptor agonists (GLP-1RA) are associated with effective glycemic and body weight control in type 2 diabetes (T2DM), however, not all patients achieve their respective targets with GLP-1 RA therapy [1]. LY3298176 is a 39-amino acid synthetic peptide with agonist activity at both the glucose-dependent insulin-tropic polypeptide (GIP) and GLP-1 receptors, to be administered subcutaneously, once-weekly [2]. This randomized, double-blind, phase 2 study evaluated the dose-response relationship of LY3298176 (1, 5, 10, and 15 mg) in T2DM patients, and assessed the efficacy and safety in comparison with placebo and the GLP-1RA dulaglutide 1.5 mg.
For this purpose, 316 adult patients (aged 18-75) with T2DM for at least 6 months that was inadequately controlled with diet and exercise alone or with stable metformin therapy for at least 3 months before screening, and with a BMI of 23–50 kg/m² were randomly allocated (1:1:1:1:1:1) to one of the six parallel treatment groups for 26 weeks (placebo, LY3298176 1, 5, 10, and 15 mg, and dulaglutide 1.5 mg).
The primary efficacy outcome was change in HbA1c from baseline to 26 weeks in the modified intention-to-treat (mITT) population, defined as all participants who took at least one dose of study drug and had at least one post-baseline measurement of any outcome.
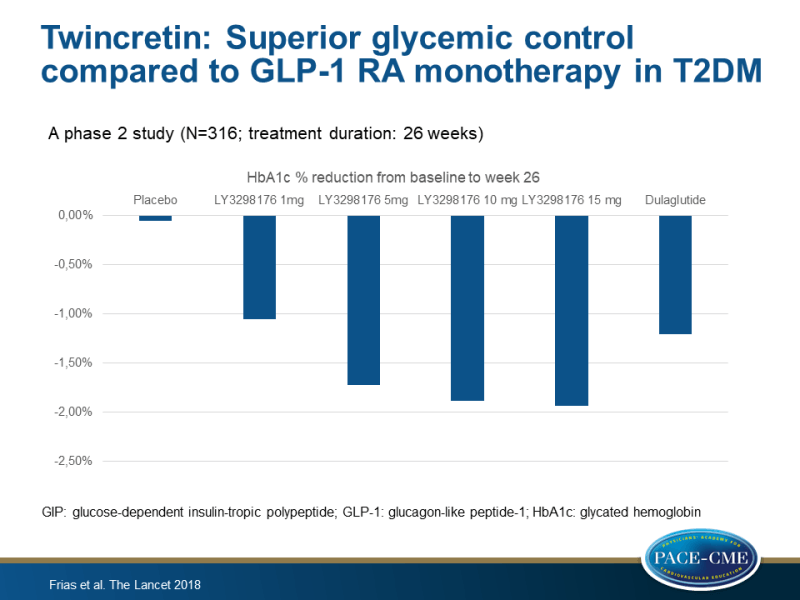
Main results
- The mean changes in HbA1c from baseline to week 26 with LY3298176 were –1.06% for 1 mg, –1.73% for 5 mg, –1.89% for 10 mg, and –1.94% for 15 mg, compared with –0.06% for placebo, and with –1.21% for dulaglutide.
- At week 26, 33–90% of patients treated with LY3298176 achieved the HbA1c target of less than 7.0% (vs 52% with dulaglutide and 12% with placebo), and 15–82% achieved the HbA1c target of at least 6.5% (vs 39% with dulaglutide and 2% with placebo). These proportions of participants were largest with LY3298176 15 mg compared to dulaglutide (90.0%, P<0.0001 and 82.0%, P<0.0001, respectively).
- Changes in fasting plasma glucose from baseline to week 26 ranged from –0.4 mmol/L (–6.8 mg/dL) to –3.4 mmol/L (–60.7 mg/dL) for the LY3298176 groups, compared with 0.9 mmol/L (15.5 mg/dL) for placebo and –1.2 mmol/L (–21.2 mg/dL) for dulaglutide. Largest change in fasting plasma glucose was observed with LY3298176 10 mg.
- Changes in mean bodyweight from baseline to week 26 ranged from –0.9 kg to –11.3 kg for the LY3298176 groups compared with –0.4 kg for placebo and –2.7 kg for dulaglutide. All doses of LY3298176 reduced bodyweight relative to placebo in a dose-dependent manner, with greater reduction in bodyweight observed with 5 mg, 10 mg, and 15 mg LY3298176 compared with dulaglutide.
- At week 26, 14–71% of those treated with LY3298176 achieved the weight loss target of at least 5% (vs 22% with dulaglutide and 0% with placebo) and 6–39% achieved the weight loss target of at least 10% (vs 9% with dulaglutide and 0% with placebo). These proportions of participants were largest with LY3298176 15 mg compared to dulaglutide (70.6%, P<0.001 and 39.2%, P=0.0010, respectively)
- Changes in mean waist circumference from baseline to week 26 ranged from –2.1 cm to –10.2 cm for the LY3298176 groups with largest change observed after treatment with LY3298176 15 mg, compared with –1.3 cm for placebo and –2.5 cm for dulaglutide.
- Changes in mean total cholesterol from baseline to week 26 ranged from 0.2 mmol/L to –0.3 mmol/L for LY3298176 compared with 0.3 mmol/L for placebo and –0.2 mmol/L for dulaglutide.
- In the LY3298176 groups, the number of adverse events increased in a dose-dependent manner, which was largely driven by the increasing incidence of mild or moderate gastro-intestinal adverse events (LY3298176: 23.1-66.0%, dulaglutide: 42.6% and placebo: 9.8%). There were no reports of severe hypoglycemia.
Conclusion
In this phase 2b study, LY3298176, a dual GIP and GLP-1 receptor agonist, led to a statistically significant and clinically meaningful dose-dependent improvement of glucose lowering and body weight reduction compared with dulaglutide and placebo
Editorial comment
In their editorial article, Stumvoll and Tschöp [4] note that it is too early for any far-reaching clinical conclusion or recommendation based on the results of the study of Frias et al.. Several issues need to be addressed, for example whether LY3298176 is also superior to semaglutide, the most effective GLP-1 receptor agonist, which patients will benefit most from the dual agonist, and what will be the optimal dosing. As the field moves toward a future of metabolic precision medicine, future decision-making processes might include identifying the best incretin-based therapy for any individual patient from a choice of single molecule combination therapeutics. Better understanding of respective mechanisms of action and reliable predictive markers will be of considerable value to appropriately personalize such therapeutic choices. The authors conclude: ‘The joint interpretation of encouraging results from pharmacologically optimized GLP-1 monoagonism and these new observations indicating superior benefits of a twincretin approach suggest that these treatment approaches could contribute to efforts to reduce the rising prevalence of obesity and type 2 diabetes.’
References
1. Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol 2016; 4: 525–36.
2. Tamer C, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab (in press).
3. Stumvoll M and Tschöp M. Twice the benefits with twincretins? The Lancet 2018; published online ahead of print.
