Type of existing CVD at baseline affects CV benefits of SGLT2 inhibition
A meta-analysis of three CVOTs showed moderate benefits of SGLT2i on atherosclerotic MACE only in those with ASCVD, whereas robust benefits on HHF and renal disease were observed regardless of baseline CVD or HF.
SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trialsLiterature - Zelniker TA, Wiviott SD, Raz I et al. - The Lancet 2018: published online ahead of print
Introduction and methods
Although sodium-glucose cotransporter-2 inhibitors (SGLT2i) are recommended to reduce risk of MACE in patients with atherosclerotic cardiovascular disease (ASCVD) but not multiple risk factors [1,2], it remains unknown whether the CV efficacy of SGLT2i depends on baseline ASCVD risk categories, a history of HF and renal function. Publication of the DECLARE-TIMI 58 results [3] now allows betters investigation of the potential heterogeneity CV efficacy by baseline CVD risk. Moreover, a meta-analysis allows better assessment of some infrequent adverse events that have been observed in some of the SGLT2i CVOTs.
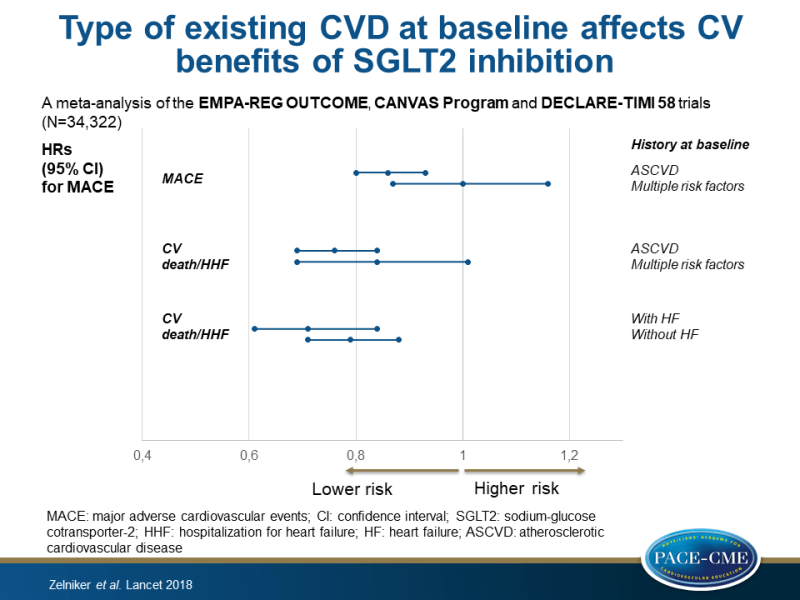
Therefore, this meta-analysis combined data from all large-scale placebo-controlled CVOTs of SGLT2i published up to Sept, 2018 (EMPA-REG OUTCOME, CANVAS Program and DECLARE-TIMI 58 trials; n=34,322) to gain more reliable estimates of the efficacy and safety of specific outcomes overall and in relevant subgroups. The DECLARE-TIMI 58 also included patients without known ASCVD but with multiple risk factors (59%), and in the pooled cohort this group represented 39.8%. These subgroups consisted of: established ASCVD vs. multiple risk factors, with vs. without a history of HF, and of eGFR levels (low: <60 mL/min/1.73m², middle: 60-90 mL/min/1.73m², and high: ≥90 mL/min/1.73m²).
Efficacy outcomes were MACE, the composite of CV death or hospitalization for HF (HHF), their individual components, and a standardized composite of renal outcomes including worsening eGFR, end-stage renal disease, or renal death. Safety endpoints were non-traumatic lower limb amputations, fractures, and diabetic ketoacidosis.
Main results
Efficacy outcomes: different subgroups
- Overall, SGLT2i reduced the risk of MACE by 11% (HR: 0.89, 95%CI: 0.83-0.96, P=0.0014). When stratifying for history of ASCVD, this effect was only observed in patients with ASCVD (HR: 0.86, 95%CI: 0.80-0.93, P=0.0002), but not in patients with multiple risk factors (HR: 1.00, 95%CI: 0.87-1.16, P=0.98) (P-interaction=0.0501).
- In patients with ASCVD, SGLT2i reduced the risk of MI (HR: 0.85, 95%CI: 0.76-0.95) and CV death (HR: 0.80, 95%CI: 0.71-0.91), whereas no effect was seen in patients without ASCVD (HR: 0.80, 95%CI: 0.71-0.91). In both groups, SGLT2 inhibition did not significantly affect stroke.
- Overall, SGLT2i significantly reduced the risk for the composite of CV death or HHF by 23% (HR: 0.77, 95%CI: 0.71-0.84, P<0.0001). In patients with ASCVD, the HR for the composite of CV death or HHF was 0.76 (95%CI: 0.69-0.84, P<0.0001) and in patients with multiple risk factors it was 0.84 (95%CI: 0.69-1.01, P=0.0634) (P-interaction=0.41).
- The reduction in the composite of CV death or HHF was not statistically significantly different in patients with (HR: 0.71, 95%CI: 0.61-0.84, P<0.0001) or without (HR: 0.79, 95%CI: 0.71-0.88, P<0.0001) a history of HF at baseline (P-interaction=0.51), nor were the individual component outcomes.
- Overall, SGLT2i were renoprotective and reduced the composite of worsening of renal function, end-stage renal disease, or renal death by 45% (HR: 0.55, 95%CI: 0.48-0.64, P<0.0001), which was similar in patients with ASCVD (HR: 0.56, 95%CI: 0.47-0.67, P<0.0001) and in those with multiple risk factors (HR: 0.54, 95%CI: 0.42-0.71,P<0.0001) (P-interaction=0.71).
- The reduction in the composite renal endpoint was present across all baseline eGFR levels, but it was greatest in those with preserved renal function at baseline, with a 33% reduction in the low eGFR group (HR: 0.67, 95%CI: 0.51-0.89, P=0.0054), 44% in the middle group (HR: 0.56, 95%CI: 0.46-0.70, P<0.0001), and 56% in the highest group (HR: 0.44, 95%CI: 0.32-0.59, P<0.0001) (P-trend=0.0258).
- The reduction in HHF was 40% in the lowest eGFR group (HR: 0.60, 95%CI: 0.47-0.77, P<0.0001), 31% in the middle group (HR: 0.69, 95%CI: 0.57-0.83, P<0.0001), and a non-significant 12% in the highest group (HR: 0.88, 95%CI: 0.68-1.13, P=0.31) (P-trend=0.0073).
- The effect of baseline eGFR on MACE was less clear, and showed a non-significant trend (P=0.23), towards a higher treatment benefit in the lowest eGFR category.
Safety outcomes
- Significant heterogeneity was observed among the trials for amputations (I²=79.1%) and fractures (I²=78.2%), with an increased risk seen only in one trial.
- Diabetic ketoacidosis showed a consistently increased risk in patients treated with SGLT2i compared to placebo (HR: 2.20, 95%CI: 1.25-3.87, P=0.0060), but event rates were lower than 1 per 1000 patient-years.
Conclusion
This meta-analysis of the EMPA-REG OUTCOME, CANVAS Program and DECLARE-TIMI 58 trials showed moderate benefits of SGLT2i on atherosclerotic MACE only in those with established ASCVD. In contrast, robust reductions in HHF and progression of renal disease were observed regardless of baseline atherosclerotic risk category or a history of HF.
References
1. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016; 37: 2315–81.
2. American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2018. Diabetes Care 2018; 41 (suppl 1): S73–85.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New Engl J Med (in press).
