Unanticipated attenuation of LDL-c lowering response to humanised PCSK9 antibody over time
ACC 2017 The SPIRE trial data show that treatment with bococizumab resulted in LDL-c lowering that was not stable over time, due to generation of anti-drug antibodies.
Safety and Cardiovascular Event Efficacy of Bococizumab Among 27,000 High Risk PatientsNews - Mar. 16, 2017
Background
The SPIRE bococizumab clinical development program consisted of 6 SPIRE Lipid-lowering trials (n=4449, SPIRE HR, SPIRE LDL, SPIRE FH, SPIRE LL, SPIRE SI and SPIRE AI) and two SPIRE CV outcomes trials SPIRE-1 (n=16817) and SPIRE-2 (n=10621). The difference between SPIRE-1 and SPIRE-2 was the minimally required LDL-c level for inclusion: ≥70 mg/dL for SPIRE-1 and LDL-c ≥100 mg/dL for SPIRE-2.
In the SPIRE trials, patients on maximally tolerated statins and at high CV risk were randomised to bococizumab 150 mg SC Q2W or placebo. Bococizumab is a humanised antibody (over 90% human) directed against PCSK9.
Main results
SPIRE lipid-lowering trials
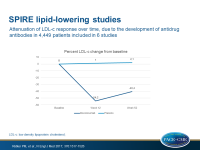
- All separate trials showed an unanticipated attenuation of LDL-c reduction at 52 weeks. Overall, 55.2% reduction was seen at 12 weeks, and 42.5% at 52 weeks.
- An increasing number of patients developed antidrug antibodies (ADAs) over time, up to 48% at the end of study. ADA development was not associated with adverse events, but there was a relation with LDL-c levels. Patients who showed higher levels of ADAs showed stronger attenuation of LDL-c reduction, and those who did not show ADAs showed only a mild attenuation of LDL-c reduction over time.
- ADA titer-dependent reductions in bococizumab concentration were seen, likely due to increased target-mediated clearance of unbound bococizumab and accelerated clearance of ADA bound bococizumab.
- A large individual variation in percent change in LDL-c at 52 weeks was observed in compliant patients on bococizumab treatment, even among those who were ADA-negative. 9% of 780 patients showed no LDL-c reduction, 31% showed a less than 50% reduction, and 60% showed at least 50% reduction.
Based on the completed SPIRE lipid-lowering trials, it was decided on November 1, 2016 to discontinue further development of bococizumab. Consequently, the SPIRE-1 and SPIRE-2 outcome trials were discontinued prematurely.
SPIRE-1 and SPIRE-2 outcome trials
- Mean baseline LDL-c was 94 mg/dL in SPIRE-1 for both bococizumab and placebo, and in SPIRE-2 it was 134 and 133 mg/dL, respectively. Absolute risk also differed between trials: 3.02 per 100 person-years (PY) in SPIRE-1 and 4.19 per 100 PY in SPIRE-2.
- Data of SPIRE-1 and SPIRE-2 confirmed the attenuation of LDL-c reduction over time.
- Data of SPIRE-1 and SPIRE-2 confirmed the wide individual variability in percent LDL-c reduction.
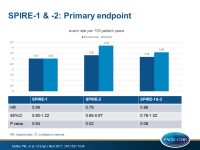
- In SPIRE 1, over a median follow-up of 7 months, 173 events were seen with bococizumab, and 173 in the placebo group (HR: 0.99, 95%CI: 0.80-1.22, P=0.94).
- In SPIRE 2, over a median follow-up of 12 months, 179 events were seen with bococizumab, and 224 in the placebo group (HR: 0.79, 95%CI: 0.65-0.97, P=0.021).
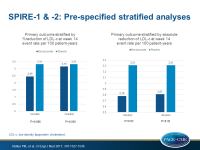
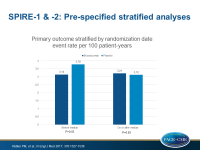
- In a prespecified analysis that combined data of both trials, stratified by magnitude of LDL-c reduction, a significant event reduction was seen in those who showed at least the median % LDL-c reduction (HR: 0.75, 95%CI: 0.61-0.92, P=0.006), but not in those with less than the median reduction (HR: 0.94, 95%CI: 0.77-1.14, P=0.51).
- In a prespecified analysis combining data of both trials, stratified by duration of bococizumab exposure, a significant event reduction was seen in those with longer duration (mean exposure period: 13.6 months, HR: 0.83, 95%CI: 0.70-0.98, P=0.028), but not in those with shorter exposure (mean: 5.6 months, HR: 1.03, 95%CI: 0.78-1.35, P=0.83).
- A larger proportion of patients discontinued bococizumab (6.3 per 100 PY exposure) due to serious adverse events as compared with placebo (4.2 per 100 PY, incidence rate ratio 1.49, P<0.001). Most often this was due to injection site reactions (10.4 vs. 1.3 per 100 PY, ratio: 8.33, P<0.001).
Conclusion
These data show that the PCSK9 antibody bococizumab lowers LDL-c by 55-60% when given in addition to statin therapy. An unanticipated attenuation of this effect was seen over time due to the development of anti-drug antibodies. This higher immunogenicity also explains the higher rate of injection site reactions observed with bococizumab, as compared with fully human monoclonal PCSK9 antibodies.
The wide individual variability in LDL-c response to bococizumab, even in those without ADAs, suggests that on-treatment LDL-c measurement is important in clinical practice. During the press conference, when the individual variability in lipid-lowering response was discussed, dr. Paul Ridker mentioned that ‘measuring LDL-c on-treatment is what our guidelines ought to say'. It is unknown whether other PCSK9 antibodies show similar individual variability.
Despite the anti-drug antibody production and the individual response and early trial termination, bococizumab significantly reduced CV event rates in the higher-risk SPIRE-2 trial, but not in the lower-risk SPIRE-1 trial. Consistent with the ‘lower the better for longer’ hypothesis, clinical benefits were greater in those who achieved and sustained greater absolute and relative reductions in LDL-c. Thus, these data support the use of PCSK9 inhibitors in selected patients in addition to aggressive statin therapy.
Disclosures
Our coverage of ACC.17 is based on the information provided during the congress.