Use of β-blockers in early pregnancy not associated with increased risk of congenital overall or cardiac malformations
In a cohort study, use of β-blockers in the first trimester was not associated with increased risk of congenital overall or cardiac malformations.
β-Blocker Use in Pregnancy and the Risk for Congenital Malformations An International Cohort StudyLiterature - Bateman BT, Heide-Jørgensen U, Einarsdottir K et al. - Annals of Internal Medicine 2018; published online ahead of print
Introduction and methods
The necessity of antihypertensive medication in early pregnancy is increasing, probably due to the higher prevalence of obesity in women of reproductive age, and older age at the time of pregnancy [1]. β-blockers are recommended in this setting, although they cross the placenta, and some data suggest a potential teratogenic effect [2].
This cohort study evaluated the association between first-trimester exposure to β-blockers in utero and congenital malformations overall and organ-specific malformations, including cardiac defects, cleft lip or palate, and central nervous system (CNS) malformations.
For this purpose, pregnancies with a diagnosis of hypertension, who received a prescription for a β-blocker during the first trimester were identified in national health registries in the five Nordic countries and the Medicaid database (MAX) in the United States through the In-PreSS (International Pregnancy Safety Study) consortium [3].
Main results
- Of 3577 women in the Nordic cohort, 19.1% were exposed to β-blockers, and of 14 900 women in the US MAX cohort, 11.2% were exposed to β-blockers in the first trimester. Labetalol was prescribed to 41.3% of exposed women in the Nordic cohort, and to 72.8% of women in the US MAX cohort.
- The RR for malformations overall with vs. without β-blocker exposure in the Nordic cohort was 1.22 (95%CI: 0.88-1.71) and in the US MAX cohort 1.01 (95%CI: 0.80-1.27).
- The RR for cardiac defects in the Nordic cohort was 0.98 (95%CI: 0.52-1.84) and in the US MAX cohort 1.16 (95%CI: 0.82-1.63).
- The RR for cleft lip or palate in the Nordic cohort was 2.26 (95%CI: 0.47-10.8) and 1.81 (95%CI: 0.52-6.33) in the US MAX cohort.
- In the US MAX cohort, the RR for CNS malformations was 1.37 (95%CI: 0.58-3.25), whereas in the Nordic cohort the number of CNS malformations was too low to allow for a meaningful analysis.
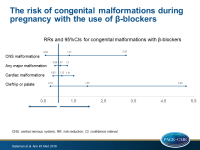
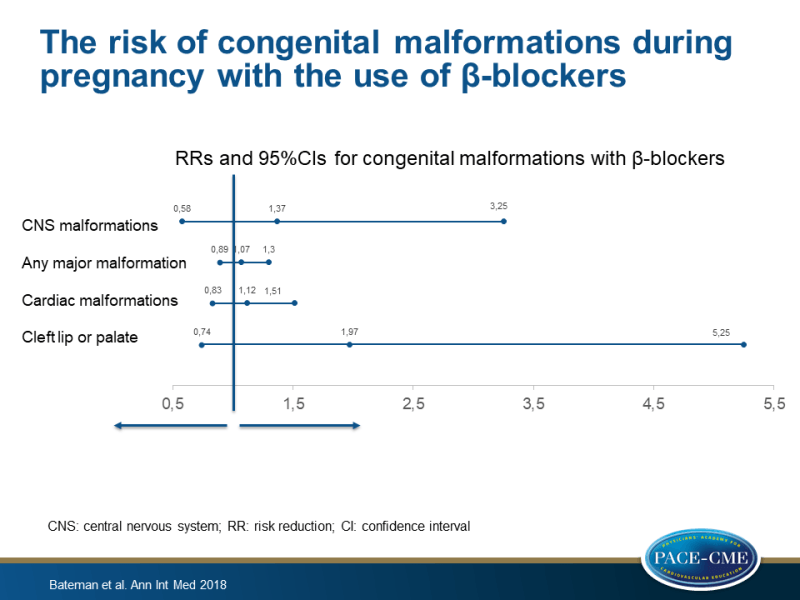
- The combined RRs of both cohorts were 1.07 (95%CI: 0.89-1.30) for any major malformation, 1.12 (95%CI: 0.83-1.51) for any cardiac malformation, and 1.97 (95%CI: 0.74-5.25) for cleft lip or palate.
Conclusion
This cohort study showed that the use of β-blockers in the first trimester of pregnancy was not associated with a large increase in the risk for congenital overall malformations or cardiac malformations. Considering low outcome numbers, an increased risk for cleft lip or palate and CNS malformations could not be excluded.
Editorial comment
In his editorial article, Ray [4] recommends the use of labetalol as a first line therapy for hypertension during pregnancy, because it has a ratio of ɑ to β blockade of 1:3, and leads to decreased peripheral vascular resistance and blood pressure, without a significant impact on heart rate and cardiac output, and unlike other conventional β-blockers, it is more effective when given at lower dosages. Ray states that ’Whatever might confound the relation between the use of a specific medication in pregnancy and an adverse perinatal outcome, maternal health remains the priority of any clinician or parent. Moreover, fetal well-being depends on maternal well-being, and untreated maternal disease both jeopardizes the health of a fetus and may shorten a pregnancy.’ The author concludes: ‘β-blockers should be used in pregnancy when indicated for the treatment of various maternal medical conditions, and labetalol should be a first-line treatment choice for chronic hypertension.’
References
1. Andrade SE, Raebel MA, Brown J, et al. Outpatient use of cardiovascular drugs during pregnancy. Pharmacoepidemiol Drug Saf. 2008;17:240-7.
2. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2011.
3. Huybrechts KF, Broms G, Christensen LB, et al. Association between methylphenidate and amphetamine use in pregnancy and risk of congenital malformations: a cohort study from the International Pregnancy Safety Study Consortium JAMA Psychiatry. 2018;75:167-75.
4. Ray JG. To β or Not to β? Very Likely OK to β. Annals of Internal Medicine 2018; published online ahead of print